Demonstration of phosphoryl group transfer indicates that the ATP-binding cassette (ABC) transporter cystic fibrosis transmembrane conductance regulator (CFTR) exhibits adenylate kinase activity
- PMID: 22948143
- PMCID: PMC3476278
- DOI: 10.1074/jbc.M112.408450
Demonstration of phosphoryl group transfer indicates that the ATP-binding cassette (ABC) transporter cystic fibrosis transmembrane conductance regulator (CFTR) exhibits adenylate kinase activity
Abstract
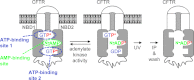
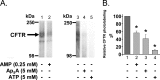
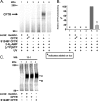
Cystic fibrosis transmembrane conductance regulator (CFTR) is a membrane-spanning adenosine 5'-triphosphate (ATP)-binding cassette (ABC) transporter. ABC transporters and other nuclear and cytoplasmic ABC proteins have ATPase activity that is coupled to their biological function. Recent studies with CFTR and two nonmembrane-bound ABC proteins, the DNA repair enzyme Rad50 and a structural maintenance of chromosome (SMC) protein, challenge the model that the function of all ABC proteins depends solely on their associated ATPase activity. Patch clamp studies indicated that in the presence of physiologically relevant concentrations of adenosine 5'-monophosphate (AMP), CFTR Cl(-) channel function is coupled to adenylate kinase activity (ATP+AMP <==> 2 ADP). Work with Rad50 and SMC showed that these enzymes catalyze both ATPase and adenylate kinase reactions. However, despite the supportive electrophysiological results with CFTR, there are no biochemical data demonstrating intrinsic adenylate kinase activity of a membrane-bound ABC transporter. We developed a biochemical assay for adenylate kinase activity, in which the radioactive γ-phosphate of a nucleotide triphosphate could transfer to a photoactivatable AMP analog. UV irradiation could then trap the (32)P on the adenylate kinase. With this assay, we discovered phosphoryl group transfer that labeled CFTR, thereby demonstrating its adenylate kinase activity. Our results also suggested that the interaction of nucleotide triphosphate with CFTR at ATP-binding site 2 is required for adenylate kinase activity. These biochemical data complement earlier biophysical studies of CFTR and indicate that the ABC transporter CFTR can function as an adenylate kinase.
Figures

Similar articles
-
Mutating the Conserved Q-loop Glutamine 1291 Selectively Disrupts Adenylate Kinase-dependent Channel Gating of the ATP-binding Cassette (ABC) Adenylate Kinase Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Reduces Channel Function in Primary Human Airway Epithelia.J Biol Chem. 2015 May 29;290(22):14140-53. doi: 10.1074/jbc.M114.611616. Epub 2015 Apr 17. J Biol Chem. 2015. PMID: 25887396 Free PMC article.
-
ATP and AMP mutually influence their interaction with the ATP-binding cassette (ABC) adenylate kinase cystic fibrosis transmembrane conductance regulator (CFTR) at separate binding sites.J Biol Chem. 2013 Sep 20;288(38):27692-27701. doi: 10.1074/jbc.M113.479675. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921386 Free PMC article.
-
An intrinsic adenylate kinase activity regulates gating of the ABC transporter CFTR.Cell. 2003 Dec 26;115(7):837-50. doi: 10.1016/s0092-8674(03)00983-8. Cell. 2003. PMID: 14697202
-
Role of CFTR's intrinsic adenylate kinase activity in gating of the Cl(-) channel.J Bioenerg Biomembr. 2007 Dec;39(5-6):473-9. doi: 10.1007/s10863-007-9119-5. J Bioenerg Biomembr. 2007. PMID: 17965924 Review.
-
Exploiting species differences to understand the CFTR Cl- channel.Biochem Soc Trans. 2015 Oct;43(5):975-82. doi: 10.1042/BST20150129. Biochem Soc Trans. 2015. PMID: 26517912 Review.
Cited by
-
AJRCCM: 100-Year Anniversary. Progress along the Pathway of Discovery Leading to Treatment and Cure of Cystic Fibrosis.Am J Respir Crit Care Med. 2017 May 1;195(9):1092-1099. doi: 10.1164/rccm.201702-0266ED. Am J Respir Crit Care Med. 2017. PMID: 28459323 Free PMC article. No abstract available.
-
Mutating the Conserved Q-loop Glutamine 1291 Selectively Disrupts Adenylate Kinase-dependent Channel Gating of the ATP-binding Cassette (ABC) Adenylate Kinase Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and Reduces Channel Function in Primary Human Airway Epithelia.J Biol Chem. 2015 May 29;290(22):14140-53. doi: 10.1074/jbc.M114.611616. Epub 2015 Apr 17. J Biol Chem. 2015. PMID: 25887396 Free PMC article.
-
ATP and AMP mutually influence their interaction with the ATP-binding cassette (ABC) adenylate kinase cystic fibrosis transmembrane conductance regulator (CFTR) at separate binding sites.J Biol Chem. 2013 Sep 20;288(38):27692-27701. doi: 10.1074/jbc.M113.479675. Epub 2013 Aug 6. J Biol Chem. 2013. PMID: 23921386 Free PMC article.
References
-
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L., et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073; Correction Science (1989) 245, 1437 - PubMed
-
- Holland I. B., Cole S. P., Kuchler K., Higgins C. F. (eds) (2003) ABC Proteins: From Bacteria to Man, 1st Ed., Academic Press, San Diego, CA
-
- Hopfner K. P., Karcher A., Shin D. S., Craig L., Arthur L. M., Carney J. P., Tainer J. A. (2000) Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

