Subtype-specific modulation of acid-sensing ion channel (ASIC) function by 2-guanidine-4-methylquinazoline
- PMID: 22948146
- PMCID: PMC3476274
- DOI: 10.1074/jbc.M112.360487
Subtype-specific modulation of acid-sensing ion channel (ASIC) function by 2-guanidine-4-methylquinazoline
Abstract
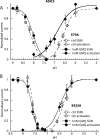
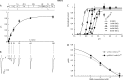
Acid-sensing ion channels (ASICs) are neuronal Na(+)-selective channels that are transiently activated by extracellular acidification. ASICs are involved in fear and anxiety, learning, neurodegeneration after ischemic stroke, and pain sensation. The small molecule 2-guanidine-4-methylquinazoline (GMQ) was recently shown to open ASIC3 at physiological pH. We have investigated the mechanisms underlying this effect and the possibility that GMQ may alter the function of other ASICs besides ASIC3. GMQ shifts the pH dependence of activation to more acidic pH in ASIC1a and ASIC1b, whereas in ASIC3 this shift goes in the opposite direction and is accompanied by a decrease in its steepness. GMQ also induces an acidic shift of the pH dependence of inactivation of ASIC1a, -1b, -2a, and -3. As a consequence, the activation and inactivation curves of ASIC3 but not other ASICs overlap in the presence of GMQ at pH 7.4, thereby creating a window current. At concentrations >1 mM, GMQ decreases maximal peak currents by reducing the unitary current amplitude. Mutation of residue Glu-79 in the palm domain of ASIC3, previously shown to be critical for channel opening by GMQ, disrupted the GMQ effects on inactivation but not activation. This suggests that this residue is involved in the consequences of GMQ binding rather than in the binding interaction itself. This study describes the mechanisms underlying the effects of a novel class of ligands that modulate the function of all ASICs as well as activate ASIC3 at physiological pH.
Figures






References
-
- Kellenberger S., Schild L. (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767 - PubMed
-
- Wemmie J. A., Price M. P., Welsh M. J. (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 29, 578–586 - PubMed
-
- Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 - PubMed
-
- Wemmie J. A., Chen J., Askwith C. C., Hruska-Hageman A. M., Price M. P., Nolan B. C., Yoder P. G., Lamani E., Hoshi T., Freeman J. H., Jr., Welsh M. J. (2002) The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34, 463–477 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

