Afadin is required for maintenance of dendritic structure and excitatory tone
- PMID: 22948147
- PMCID: PMC3476264
- DOI: 10.1074/jbc.M112.363358
Afadin is required for maintenance of dendritic structure and excitatory tone
Abstract
The dendritic field of a neuron, which is determined by both dendritic architecture and synaptic strength, defines the synaptic input of a cell. Once established, a neuron's dendritic field is thought to remain relatively stable throughout a cell's lifetime. Perturbations in a dendritic structure or excitatory tone of a cell and thus its dendritic field are cellular alterations thought to be correlated with a number of psychiatric disorders. Although several proteins are known to regulate the development of dendritic arborization, much less is known about the mechanisms that maintain dendritic morphology and synaptic strength. In this study, we find that afadin, a component of N-cadherin·β-catenin·α-N-catenin adhesion complexes, is required for the maintenance of established dendritic arborization and synapse number. We further demonstrate that afadin directly interacts with AMPA receptors and that loss of this protein reduces the surface expression of GluA1- and GluA2-AMPA receptor subunits. Collectively, these data suggest that afadin is required for the maintenance of dendritic structure and excitatory tone.
Figures

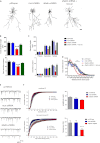
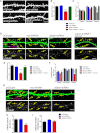

References
-
- Parrish J. Z., Emoto K., Kim M. D., Jan Y. N. (2007) Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu. Rev. Neurosci. 30, 399–423 - PubMed
-
- London M., Häusser M. (2005) Dendritic computation. Annu. Rev. Neurosci. 28, 503–532 - PubMed
-
- Spruston N. (2008) Pyramidal neurons. Dendritic structure and synaptic integration. Nat. Rev. Neurosci. 9, 206–221 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1F31MH085362/MH/NIMH NIH HHS/United States
- F31 AG031621/AG/NIA NIH HHS/United States
- T32 HD040127/HD/NICHD NIH HHS/United States
- 1F31MH087043/MH/NIMH NIH HHS/United States
- NS44322/NS/NINDS NIH HHS/United States
- F31 MH087043/MH/NIMH NIH HHS/United States
- 1F31AG031621/AG/NIA NIH HHS/United States
- F31 MH085362/MH/NIMH NIH HHS/United States
- MH097216/MH/NIMH NIH HHS/United States
- R01 MH097216/MH/NIMH NIH HHS/United States
- R01 NS044322/NS/NINDS NIH HHS/United States
- R56 MH071316/MH/NIMH NIH HHS/United States
- R01 NS071952/NS/NINDS NIH HHS/United States
- MH071316/MH/NIMH NIH HHS/United States
- R01 MH071316/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

