Characterization of conformation-dependent prion protein epitopes
- PMID: 22948149
- PMCID: PMC3481321
- DOI: 10.1074/jbc.M112.395921
Characterization of conformation-dependent prion protein epitopes
Abstract
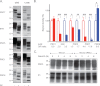
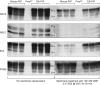
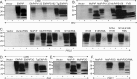
Whereas prion replication involves structural rearrangement of cellular prion protein (PrP(C)), the existence of conformational epitopes remains speculative and controversial, and PrP transformation is monitored by immunoblot detection of PrP(27-30), a protease-resistant counterpart of the pathogenic scrapie form (PrP(Sc)) of PrP. We now describe the involvement of specific amino acids in conformational determinants of novel monoclonal antibodies (mAbs) raised against randomly chimeric PrP. Epitope recognition of two mAbs depended on polymorphisms controlling disease susceptibility. Detection by one, referred to as PRC5, required alanine and asparagine at discontinuous mouse PrP residues 132 and 158, which acquire proximity when residues 126-218 form a structured globular domain. The discontinuous epitope of glycosylation-dependent mAb PRC7 also mapped within this domain at residues 154 and 185. In accordance with their conformational dependence, tertiary structure perturbations compromised recognition by PRC5, PRC7, as well as previously characterized mAbs whose epitopes also reside in the globular domain, whereas conformation-independent epitopes proximal or distal to this region were refractory to such destabilizing treatments. Our studies also address the paradox of how conformational epitopes remain functional following denaturing treatments and indicate that cellular PrP and PrP(27-30) both renature to a common structure that reconstitutes the globular domain.
Figures





Similar articles
-
Generation of monoclonal antibody that distinguishes PrPSc from PrPC and neutralizes prion infectivity.Virology. 2009 Nov 25;394(2):200-7. doi: 10.1016/j.virol.2009.08.025. Epub 2009 Sep 18. Virology. 2009. PMID: 19766283
-
Incomplete glycosylation during prion infection unmasks a prion protein epitope that facilitates prion detection and strain discrimination.J Biol Chem. 2020 Jul 24;295(30):10420-10433. doi: 10.1074/jbc.RA120.012796. Epub 2020 Jun 8. J Biol Chem. 2020. PMID: 32513872 Free PMC article.
-
Detection of prion epitopes on PrP and PrP of transmissible spongiform encephalopathies using specific monoclonal antibodies to PrP.Immunol Cell Biol. 2005 Dec;83(6):632-7. doi: 10.1111/j.1440-1711.2005.01384.x. Immunol Cell Biol. 2005. PMID: 16266315
-
The use of monoclonal antibody epitopes for tagging PrP in conversion experiments.Arch Virol Suppl. 2000;(16):285-90. doi: 10.1007/978-3-7091-6308-5_27. Arch Virol Suppl. 2000. PMID: 11214932 Review.
-
Insights into Mechanisms of Transmission and Pathogenesis from Transgenic Mouse Models of Prion Diseases.Methods Mol Biol. 2017;1658:219-252. doi: 10.1007/978-1-4939-7244-9_16. Methods Mol Biol. 2017. PMID: 28861793 Free PMC article. Review.
Cited by
-
The importance of prions.PLoS Pathog. 2013 Jan;9(1):e1003090. doi: 10.1371/journal.ppat.1003090. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382670 Free PMC article. No abstract available.
-
Detection of misfolded protein aggregates from a clinical perspective.J Clin Transl Res. 2016 Mar 22;2(1):11-26. eCollection 2016 Apr 15. J Clin Transl Res. 2016. PMID: 30873457 Free PMC article. Review.
-
Effect of poly-L-arginine in inhibiting scrapie prion protein of cultured cells.Mol Cell Biochem. 2017 Apr;428(1-2):57-66. doi: 10.1007/s11010-016-2916-6. Epub 2017 Jan 7. Mol Cell Biochem. 2017. PMID: 28063003 Free PMC article.
-
Novel Prion Strain as Cause of Chronic Wasting Disease in a Moose, Finland.Emerg Infect Dis. 2023 Feb;29(2):323-332. doi: 10.3201/eid2902.220882. Emerg Infect Dis. 2023. PMID: 36692340 Free PMC article.
-
Structural effects of PrP polymorphisms on intra- and interspecies prion transmission.Proc Natl Acad Sci U S A. 2014 Jul 29;111(30):11169-74. doi: 10.1073/pnas.1404739111. Epub 2014 Jul 17. Proc Natl Acad Sci U S A. 2014. PMID: 25034251 Free PMC article.
References
-
- Stahl N., Borchelt D. R., Hsiao K., Prusiner S. B. (1987) Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51, 229–240 - PubMed
-
- Riek R., Hornemann S., Wider G., Billeter M., Glockshuber R., Wüthrich K. (1996) NMR structure of the mouse prion protein domain PrP(121–231). Nature 382, 180–182 - PubMed
-
- Chen S. G., Teplow D. B., Parchi P., Teller J. K., Gambetti P., Autilio-Gambetti L. (1995) Truncated forms of the human prion protein in normal brain and in prion diseases. J. Biol. Chem. 270, 19173–19180 - PubMed
-
- Yadavalli R., Guttmann R. P., Seward T., Centers A. P., Williamson R. A., Telling G. C. (2004) Calpain-dependent endoproteolytic cleavage of PrPSc modulates scrapie prion propagation. J. Biol. Chem. 279, 21948–21956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/D/20251967/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20251968/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- P01 AI077774-015261/AI/NIAID NIH HHS/United States
- BBS/E/D/05241340/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 NS040334/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

