Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification
- PMID: 22948150
- PMCID: PMC3481314
- DOI: 10.1074/jbc.M112.398164
Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification
Abstract
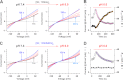
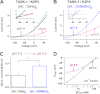
Two-pore domain K(+) channels (K2P) mediate background K(+) conductance and play a key role in a variety of cellular functions. Among the 15 mammalian K2P isoforms, TWIK-1, TASK-1, and TASK-3 K(+) channels are sensitive to extracellular acidification. Lowered or acidic extracellular pH (pH(o)) strongly inhibits outward currents through these K2P channels. However, the mechanism of how low pH(o) affects these acid-sensitive K2P channels is not well understood. Here we show that in Na(+)-based bath solutions with physiological K(+) gradients, lowered pH(o) largely shifts the reversal potential of TWIK-1, TASK-1, and TASK-3 K(+) channels, which are heterologously expressed in Chinese hamster ovary cells, into the depolarizing direction and significantly increases their Na(+) to K(+) relative permeability. Low pH(o)-induced inhibitions in these acid-sensitive K2P channels are more profound in Na(+)-based bath solutions than in channel-impermeable N-methyl-D-glucamine-based bath solutions, consistent with increases in the Na(+) to K(+) relative permeability and decreases in electrochemical driving forces of outward K(+) currents of the channels. These findings indicate that TWIK-1, TASK-1, and TASK-3 K(+) channels change ion selectivity in response to lowered pH(o), provide insights on the understanding of how extracellular acidification modulates acid-sensitive K2P channels, and imply that these acid-sensitive K2P channels may regulate cellular function with dynamic changes in their ion selectivity.
Figures



References
-
- Goldstein S. A., Bayliss D. A., Kim D., Lesage F., Plant L. D., Rajan S. (2005) International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol. Rev. 57, 527–540 - PubMed
-
- Lotshaw D. P. (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys. 47, 209–256 - PubMed
-
- Enyedi P., Czirják G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605 - PubMed
-
- Rajan S., Plant L. D., Rabin M. L., Butler M. H., Goldstein S. A. (2005) Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121, 37–47 - PubMed
-
- Cohen A., Ben-Abu Y., Hen S., Zilberberg N. (2008) A novel mechanism for human K2P2.1 channel gating: facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 283, 19448–19455 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

