Bidirectional coupling between ryanodine receptors and Ca2+ release-activated Ca2+ (CRAC) channel machinery sustains store-operated Ca2+ entry in human T lymphocytes
- PMID: 22948152
- PMCID: PMC3481322
- DOI: 10.1074/jbc.M112.398974
Bidirectional coupling between ryanodine receptors and Ca2+ release-activated Ca2+ (CRAC) channel machinery sustains store-operated Ca2+ entry in human T lymphocytes
Abstract
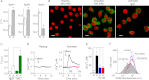
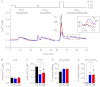
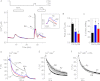
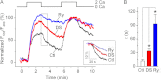
The expression and functional significance of ryanodine receptors (RyR) were investigated in resting and activated primary human T cells. RyR1, RyR2, and RyR3 transcripts were detected in human T cells. RyR1/2 transcript levels increased, whereas those of RyR3 decreased after T cell activation. RyR1/2 protein immunoreactivity was detected in activated but not in resting T cells. The RyR agonist caffeine evoked Ca(2+) release from the intracellular store in activated T cells but not in resting T cells, indicating that RyR are functionally up-regulated in activated T cells compared with resting T cells. In the presence of store-operated Ca(2+) entry (SOCE) via plasmalemmal Ca(2+) release-activated Ca(2+) (CRAC) channels, RyR blockers reduced the Ca(2+) leak from the endoplasmic reticulum (ER) and the magnitude of SOCE, suggesting that a positive feedback relationship exists between RyR and CRAC channels. Overexpression of fluorescently tagged RyR2 and stromal interaction molecule 1 (STIM1), an ER Ca(2+) sensor gating CRAC channels, in HEK293 cells revealed that RyR are co-localized with STIM1 in the puncta formed after store depletion. These data indicate that in primary human T cells, the RyR are coupled to CRAC channel machinery such that SOCE activates RyR via a Ca(2+)-induced Ca(2+) release mechanism, which in turn reduces the Ca(2+) concentration within the ER lumen in the vicinity of STIM1, thus facilitating SOCE by reducing store-dependent CRAC channel inactivation. Treatment with RyR blockers suppressed activated T cell expansion, demonstrating the functional importance of RyR in T cells.
Figures

Similar articles
-
Conformation of ryanodine receptor-2 gates store-operated calcium entry in rat pulmonary arterial myocytes.Cardiovasc Res. 2016 Jul 1;111(1):94-104. doi: 10.1093/cvr/cvw067. Epub 2016 Mar 24. Cardiovasc Res. 2016. PMID: 27013634 Free PMC article.
-
Store-operated Ca2+ influx causes Ca2+ release from the intracellular Ca2+ channels that is required for T cell activation.J Biol Chem. 2008 May 2;283(18):12512-9. doi: 10.1074/jbc.M709330200. Epub 2008 Mar 3. J Biol Chem. 2008. PMID: 18316371
-
Dental enamel cells express functional SOCE channels.Sci Rep. 2015 Oct 30;5:15803. doi: 10.1038/srep15803. Sci Rep. 2015. PMID: 26515404 Free PMC article.
-
Molecular pharmacology of store-operated CRAC channels.Channels (Austin). 2013 Sep-Oct;7(5):402-14. doi: 10.4161/chan.25292. Epub 2013 Aug 26. Channels (Austin). 2013. PMID: 23807116 Free PMC article. Review.
-
Modulation of Orai1 and STIM1 by Cellular Factors.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 4. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 4. PMID: 30299655 Free Books & Documents. Review.
Cited by
-
The Role of TRPC1 in Modulating Cancer Progression.Cells. 2020 Feb 7;9(2):388. doi: 10.3390/cells9020388. Cells. 2020. PMID: 32046188 Free PMC article. Review.
-
TRIC-A shapes oscillatory Ca2+ signals by interaction with STIM1/Orai1 complexes.PLoS Biol. 2020 Apr 24;18(4):e3000700. doi: 10.1371/journal.pbio.3000700. eCollection 2020 Apr. PLoS Biol. 2020. PMID: 32330125 Free PMC article.
-
The functional contribution of calcium ion flux heterogeneity in T cells.Immunol Cell Biol. 2015 Sep;93(8):694-704. doi: 10.1038/icb.2015.34. Epub 2015 Mar 31. Immunol Cell Biol. 2015. PMID: 25823995 Review.
-
Modulation of Ryanodine Receptors Activity Alters the Course of Experimental Autoimmune Encephalomyelitis in Mice.Front Physiol. 2021 Dec 17;12:770820. doi: 10.3389/fphys.2021.770820. eCollection 2021. Front Physiol. 2021. PMID: 35027891 Free PMC article.
-
Regulatory Network Analysis of Mutated Genes Based on Multi-Omics Data Reveals the Exclusive Features in Tumor Immune Microenvironment Between Left-Sided and Right-Sided Colon Cancer.Front Oncol. 2021 Jun 15;11:685515. doi: 10.3389/fonc.2021.685515. eCollection 2021. Front Oncol. 2021. PMID: 34211853 Free PMC article.
References
-
- Germain R. N. (2002) T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2, 309–322 - PubMed
-
- Swain S. L. (1999) Helper T cell differentiation. Curr. Opin. Immunol. 11, 180–185 - PubMed
-
- Lewis R. S. (2001) Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 - PubMed
-
- Metcalfe J. C., Pozzan T., Smith G. A., Hesketh T. R. (1980) A calcium hypothesis for the control of cell growth. Biochem. Soc. Symp. 45, 1–26 - PubMed
-
- Crabtree G. R. (1989) Contingent genetic regulatory events in T lymphocyte activation. Science 243, 355–361 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

