Engineering of sialylated mucin-type O-glycosylation in plants
- PMID: 22948156
- PMCID: PMC3476317
- DOI: 10.1074/jbc.M112.402685
Engineering of sialylated mucin-type O-glycosylation in plants
Abstract
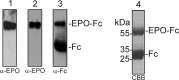
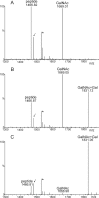
Proper N- and O-glycosylation of recombinant proteins is important for their biological function. Although the N-glycan processing pathway of different expression hosts has been successfully modified in the past, comparatively little attention has been paid to the generation of customized O-linked glycans. Plants are attractive hosts for engineering of O-glycosylation steps, as they contain no endogenous glycosyltransferases that perform mammalian-type Ser/Thr glycosylation and could interfere with the production of defined O-glycans. Here, we produced mucin-type O-GalNAc and core 1 O-linked glycan structures on recombinant human erythropoietin fused to an IgG heavy chain fragment (EPO-Fc) by transient expression in Nicotiana benthamiana plants. Furthermore, for the generation of sialylated core 1 structures constructs encoding human polypeptide:N-acetylgalactosaminyltransferase 2, Drosophila melanogaster core 1 β1,3-galactosyltransferase, human α2,3-sialyltransferase, and Mus musculus α2,6-sialyltransferase were transiently co-expressed in N. benthamiana together with EPO-Fc and the machinery for sialylation of N-glycans. The formation of significant amounts of mono- and disialylated O-linked glycans was confirmed by liquid chromatography-electrospray ionization-mass spectrometry. Analysis of the three EPO glycopeptides carrying N-glycans revealed the presence of biantennary structures with terminal sialic acid residues. Our data demonstrate that N. benthamiana plants are amenable to engineering of the O-glycosylation pathway and can produce well defined human-type O- and N-linked glycans on recombinant therapeutics.
Figures




Similar articles
-
Engineering of human-type O-glycosylation in Nicotiana benthamiana plants.Bioengineered. 2013 Jul-Aug;4(4):191-6. doi: 10.4161/bioe.22857. Epub 2012 Nov 12. Bioengineered. 2013. PMID: 23147167 Free PMC article.
-
Engineering of N. benthamiana L. plants for production of N-acetylgalactosamine-glycosylated proteins--towards development of a plant-based platform for production of protein therapeutics with mucin type O-glycosylation.BMC Biotechnol. 2010 Aug 24;10:62. doi: 10.1186/1472-6750-10-62. BMC Biotechnol. 2010. PMID: 20735851 Free PMC article.
-
N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans.Glycobiology. 2011 Jun;21(6):813-23. doi: 10.1093/glycob/cwr009. Epub 2011 Feb 11. Glycobiology. 2011. PMID: 21317243 Free PMC article.
-
Myriad mechanisms: factors regulating the synthesis of aberrant mucin-type O-glycosylation found on cancer cells.Glycobiology. 2025 Apr 23;35(6):cwaf023. doi: 10.1093/glycob/cwaf023. Glycobiology. 2025. PMID: 40247681 Review.
-
Glycoproteins from insect cells: sialylated or not?Biol Chem. 2001 Feb;382(2):151-9. doi: 10.1515/BC.2001.023. Biol Chem. 2001. PMID: 11308014 Free PMC article. Review.
Cited by
-
β-Hexosaminidases Along the Secretory Pathway of Nicotiana benthamiana Have Distinct Specificities Toward Engineered Helminth N-Glycans on Recombinant Glycoproteins.Front Plant Sci. 2021 Mar 17;12:638454. doi: 10.3389/fpls.2021.638454. eCollection 2021. Front Plant Sci. 2021. PMID: 33815445 Free PMC article.
-
A gene responsible for prolyl-hydroxylation of moss-produced recombinant human erythropoietin.Sci Rep. 2013 Oct 22;3:3019. doi: 10.1038/srep03019. Sci Rep. 2013. PMID: 24145658 Free PMC article.
-
Implications of O-glycan modifications in the hinge region of a plant-produced SARS-CoV-2-IgA antibody on functionality.Front Bioeng Biotechnol. 2024 Mar 6;12:1329018. doi: 10.3389/fbioe.2024.1329018. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38511130 Free PMC article.
-
Production and glyco-engineering of immunomodulatory helminth glycoproteins in plants.Sci Rep. 2017 Apr 10;7:45910. doi: 10.1038/srep45910. Sci Rep. 2017. PMID: 28393916 Free PMC article.
-
Glyco-engineering for the production of recombinant IgA1 with distinct mucin-type O-glycans in plants.Bioengineered. 2016 Nov;7(6):484-489. doi: 10.1080/21655979.2016.1201251. Epub 2016 Jun 22. Bioengineered. 2016. PMID: 27333379 Free PMC article.
References
-
- Zimran A., Brill-Almon E., Chertkoff R., Petakov M., Blanco-Favela F., Muñoz E. T., Solorio-Meza S. E., Amato D., Duran G., Giona F., Heitner R., Rosenbaum H., Giraldo P., Mehta A., Park G., Phillips M., Elstein D., Altarescu G., Szleifer M., Hashmueli S., Aviezer D. (2011) Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood 118, 5767–5773 - PubMed
-
- Maxmen A. (2012) Drug-making plant blooms. Nature 485, 160. - PubMed
-
- Ghaderi D., Zhang M., Hurtado-Ziola N., Varki A. (2012) Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol. Genet. Eng. Rev. 28, 147–175 - PubMed
-
- Jefferis R. (2012) Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 526, 159–166 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

