Phenotypic screening reveals topoisomerase I as a breast cancer stem cell therapeutic target
- PMID: 22948175
- PMCID: PMC3660065
- DOI: 10.18632/oncotarget.632
Phenotypic screening reveals topoisomerase I as a breast cancer stem cell therapeutic target
Abstract
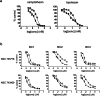
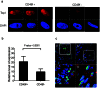
Cancer stem cells (CSCs) are a subpopulation generally thought to be responsible for cancer initiation and progression. Because CSCs are often rare in the total tumor cell population and differentiate rapidly when grown in culture, it has been challenging to uncover compounds that selectively target CSCs. We previously described CSC-emulating cells derived from breast cancer cell lines that maintained a stable undifferentiated state. We optimized a phenotypic assay with these cells and screened 1,280-bioactive compounds, identifying five that preferentially inhibited CSC-like cell proliferation. Using a compound-guided target identification approach, we found high topoisomerase I (Topo I) expression levels in breast CSC-like cells and primary breast CSCs. Structurally unrelated small molecules targeting Topo I preferentially inhibited CSC-like cells. These results illustrate the substantial power of this CSC phenotypic screening platform and promote Topo I as a potential molecular therapeutic target for therapies aimed at expunging CSCs.
Figures


References
-
- Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008;18:48–53. - PubMed
-
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. - PubMed
-
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. - PubMed
-
- Wong DJ, Segal E, Chang HY. Stemness, cancer and cancer stem cells. Cell Cycle. 2008;7:3622–3624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

