Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway
- PMID: 22948212
- PMCID: PMC3473203
- DOI: 10.1210/en.2012-1572
Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway
Abstract
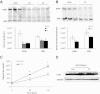
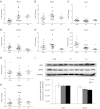
Although studies in vitro and in hypothyroid animals show that thyroid hormone can, under some circumstances, modulate the actions of low-density lipoprotein (LDL) receptors, the mechanisms responsible for thyroid hormone's lipid-lowering effects are not completely understood. We tested whether LDL receptor (LDLR) expression was required for cholesterol reduction by treating control and LDLR-knockout mice with two forms of thyroid hormone T(3) and 3,5-diiodo-l-thyronine. High doses of both 3,5-diiodo-l-thyronine and T(3) dramatically reduced circulating total and very low-density lipoprotein/LDL cholesterol (∼70%) and were associated with reduced plasma T(4) level. The cholesterol reduction was especially evident in the LDLR-knockout mice. Circulating levels of both apolipoprotein B (apo)B48 and apoB100 were decreased. Surprisingly, this reduction was not associated with increased protein or mRNA expression of the hepatic lipoprotein receptors LDLR-related protein 1 or scavenger receptor-B1. Liver production of apoB was markedly reduced, whereas triglyceride production was increased. Thus, thyroid hormones reduce apoB lipoproteins via a non-LDLR pathway that leads to decreased liver apoB production. This suggests that drugs that operate in a similar manner could be a new therapy for patients with genetic defects in the LDLR.
Figures
Similar articles
-
Lipoprotein clearance mechanisms in LDL receptor-deficient "Apo-B48-only" and "Apo-B100-only" mice.J Clin Invest. 1998 Oct 15;102(8):1559-68. doi: 10.1172/JCI4164. J Clin Invest. 1998. PMID: 9788969 Free PMC article.
-
LDL Receptor Regulates the Reverse Transport of Macrophage-Derived Unesterified Cholesterol via Concerted Action of the HDL-LDL Axis: Insight From Mouse Models.Circ Res. 2020 Aug 28;127(6):778-792. doi: 10.1161/CIRCRESAHA.119.316424. Epub 2020 Jun 4. Circ Res. 2020. PMID: 32495699
-
Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes.Diabetes. 2008 Jun;57(6):1674-82. doi: 10.2337/db08-0083. Epub 2008 Mar 17. Diabetes. 2008. PMID: 18346984
-
Lipoprotein size and atherosclerosis susceptibility in Apoe(-/-) and Ldlr(-/-) mice.Arterioscler Thromb Vasc Biol. 2001 Oct;21(10):1567-70. doi: 10.1161/hq1001.097780. Arterioscler Thromb Vasc Biol. 2001. PMID: 11597927 Review.
-
ApoB metabolism in familial hypercholesterolemia. Inconsistencies with the LDL receptor paradigm.Arterioscler Thromb. 1994 Apr;14(4):501-10. doi: 10.1161/01.atv.14.4.501. Arterioscler Thromb. 1994. PMID: 8148348 Review.
Cited by
-
An Overview of Cardiovascular Risk in Pituitary Disorders.Medicina (Kaunas). 2024 Jul 30;60(8):1241. doi: 10.3390/medicina60081241. Medicina (Kaunas). 2024. PMID: 39202522 Free PMC article. Review.
-
Triglyceride Mobilization from Lipid Droplets Sustains the Anti-Steatotic Action of Iodothyronines in Cultured Rat Hepatocytes.Front Physiol. 2016 Jan 12;6:418. doi: 10.3389/fphys.2015.00418. eCollection 2015. Front Physiol. 2016. PMID: 26793120 Free PMC article.
-
The effects of 3,5-diiodothyronine on energy balance.Front Physiol. 2015 Jan 13;5:528. doi: 10.3389/fphys.2014.00528. eCollection 2014. Front Physiol. 2015. PMID: 25628573 Free PMC article. No abstract available.
-
3,5-Diiodo-L-Thyronine Exerts Metabolically Favorable Effects on Visceral Adipose Tissue of Rats Receiving a High-Fat Diet.Nutrients. 2019 Jan 27;11(2):278. doi: 10.3390/nu11020278. Nutrients. 2019. PMID: 30691227 Free PMC article.
-
Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists.Thyroid. 2019 Sep;29(9):1173-1191. doi: 10.1089/thy.2018.0664. Thyroid. 2019. PMID: 31389309 Free PMC article. Review.
References
-
- Mason RL, Hunt HM, Hurzthal L. 1930. Blood cholesterol values in hyperthyroidism and hypothyroidism-their significance. N Engl J Med 26:1273–1278
-
- Pramfalk C, Pedrelli M, Parini P. 2011. Role of thyroid receptor β in lipid metabolism. Biochim Biophys Acta 1812:929–937 - PubMed
-
- Faber J, Kirkegaard C, Lumholtz IB, Siersbaek-Nielsen K, Friis T. 1982. Simultaneous measurement of 3,5-diiodothyronine and 3,5,3′-triiodothyronine turnover kinetics in euthyroid hyperthyroid, and hypothyroid subjects. J Clin Endocrinol Metab 55:8–12 - PubMed
-
- McClure J, De Mowbray RR, Gilliland IC. 1961. The effect of 3:5-diiodo-D-thyronine on serum cholesterol. J Endocrinol 22:87–93 - PubMed
-
- Goglia F. 2005. Biological effects of 3,5-diiodothyronine (T2). Biochemistry (Mosc) 70:164–172 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

