Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice
- PMID: 22948219
- PMCID: PMC3473198
- DOI: 10.1210/en.2012-1522
Testosterone and 17β-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice
Abstract
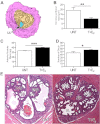
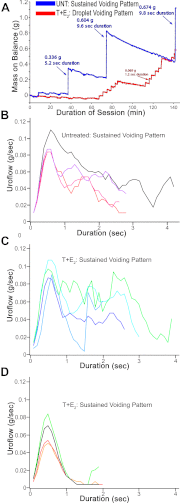
Benign prostatic hyperplasia (BPH) and bladder outlet obstruction (BOO) are common in older men and can contribute to lower urinary tract symptoms that significantly impact quality of life. Few existing models of BOO and BPH use physiological levels of hormones associated with disease progression in humans in a genetically manipulable organism. We present a model of BPH and BOO induced in mice with testosterone (T) and 17β-estradiol (E(2)). Male mice were surgically implanted with slow-releasing sc pellets containing 25 mg T and 2.5 mg E(2) (T+E(2)). After 2 and 4 months of hormone treatment, we evaluated voiding patterns and examined the gross morphology and histology of the bladder, urethra, and prostate. Mice treated with T+E(2) developed significantly larger bladders than untreated mice, consistent with BOO. Some mice treated with T+E(2) had complications in the form of bladder hypertrophy, diverticula, calculi, and eventual decompensation with hydronephrosis. Hormone treatment caused a significant decrease in the size of the urethral lumen, increased prostate mass, and increased number of prostatic ducts associated with the prostatic urethra, compared with untreated mice. Voiding dysfunction was observed in mice treated with T+E(2), who exhibited droplet voiding pattern with significantly decreased void mass, shorter void duration, and fewer sustained voids. The constellation of lower urinary tract abnormalities, including BOO, enlarged prostates, and voiding dysfunction seen in male mice treated with T+E(2) is consistent with BPH in men. This model is suitable for better understanding molecular mechanisms and for developing novel strategies to address BPH and BOO.
Figures


Similar articles
-
Increased susceptibility of estrogen-induced bladder outlet obstruction in a novel mouse model.Lab Invest. 2015 May;95(5):546-60. doi: 10.1038/labinvest.2015.30. Epub 2015 Feb 23. Lab Invest. 2015. PMID: 25706094
-
Urodynamic Features and Significant Predictors of Bladder Outlet Obstruction in Patients With Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia and Small Prostate Volume.Urology. 2016 Mar;89:96-102. doi: 10.1016/j.urology.2015.11.027. Epub 2015 Dec 9. Urology. 2016. PMID: 26683755
-
The effect of sildenafil citrate on bladder outlet obstruction: a mouse model.BJU Int. 2009 Jul;104(2):252-6. doi: 10.1111/j.1464-410X.2008.08324.x. Epub 2008 Dec 19. BJU Int. 2009. PMID: 19154466
-
Detrusor underactivity in men with lower urinary tract symptoms/benign prostatic obstruction: characterization and potential impact on indications for surgical treatment of the prostate.Curr Opin Urol. 2016 Jan;26(1):3-10. doi: 10.1097/MOU.0000000000000246. Curr Opin Urol. 2016. PMID: 26574876 Review.
-
[Consequences of prostatic obstruction on bladder function, impact of removal, and management of recurrence after surgery].Prog Urol. 2018 Nov;28(15):813-820. doi: 10.1016/j.purol.2018.08.013. Epub 2018 Sep 24. Prog Urol. 2018. PMID: 30262261 Review. French.
Cited by
-
Polychlorinated biphenyl (PCB) exposure in adult female mice can influence bladder contractility.Am J Clin Exp Urol. 2023 Oct 15;11(5):367-384. eCollection 2023. Am J Clin Exp Urol. 2023. PMID: 37941647 Free PMC article.
-
Expression and colocalization of β-catenin and lymphoid enhancing factor-1 in prostate cancer progression.Hum Pathol. 2016 May;51:124-33. doi: 10.1016/j.humpath.2015.12.024. Epub 2016 Jan 19. Hum Pathol. 2016. PMID: 27067790 Free PMC article.
-
Review of Prostate Anatomy and Embryology and the Etiology of Benign Prostatic Hyperplasia.Urol Clin North Am. 2016 Aug;43(3):279-88. doi: 10.1016/j.ucl.2016.04.012. Urol Clin North Am. 2016. PMID: 27476121 Free PMC article. Review.
-
Estrogens and Male Lower Urinary Tract Dysfunction.Curr Urol Rep. 2015 Sep;16(9):61. doi: 10.1007/s11934-015-0534-6. Curr Urol Rep. 2015. PMID: 26156791 Free PMC article. Review.
-
Reproductive tract biology: Of mice and men.Differentiation. 2019 Nov-Dec;110:49-63. doi: 10.1016/j.diff.2019.07.004. Epub 2019 Sep 6. Differentiation. 2019. PMID: 31622789 Free PMC article. Review.
References
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. 1984. The development of human benign prostatic hyperplasia with age. J Urol 132:474–479 - PubMed
-
- Oelke M, Kirschner-Hermanns R, Thiruchelvam N, Heesakkers J. 2012. Can we identify men who will have complications from benign prostatic obstruction (BPO)?: ICI-RS 2011. Neurourol Urodyn 31:322–326 - PubMed
-
- Irwin DE, Milsom I, Kopp Z, Abrams P, Artibani W, Herschorn S. 2009. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: impact of overactive bladder. Eur Urol 56:14–20 - PubMed
-
- Welch G, Weinger K, Barry MJ. 2002. Quality-of-life impact of lower urinary tract symptom severity: results from the Health Professionals Follow-up Study. Urology 59:245–250 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM07356/GM/NIGMS NIH HHS/United States
- RC2ESO18764/RC/CCR NIH HHS/United States
- T32 GM007356/GM/NIGMS NIH HHS/United States
- R01 CA123199/CA/NCI NIH HHS/United States
- R01 DK075036/DK/NIDDK NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- R01HL62572/HL/NHLBI NIH HHS/United States
- CA140217/CA/NCI NIH HHS/United States
- R01 CA140217/CA/NCI NIH HHS/United States
- UL1 TR000427/TR/NCATS NIH HHS/United States
- R01CA123199/CA/NCI NIH HHS/United States
- R01DK091193/DK/NIDDK NIH HHS/United States
- R01 DK091193/DK/NIDDK NIH HHS/United States
- RC2 ES018764/ES/NIEHS NIH HHS/United States
- R01DK075036/DK/NIDDK NIH HHS/United States
- 1UL1RR025011/RR/NCRR NIH HHS/United States
- R01DK093690/DK/NIDDK NIH HHS/United States
- R01 HL062572/HL/NHLBI NIH HHS/United States
- R01 DK093690/DK/NIDDK NIH HHS/United States
- 9U54TR000021./TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

