Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits
- PMID: 22948462
- PMCID: PMC3416900
- DOI: 10.6061/clinics/2012(08)13
Vitreous pharmacokinetics and electroretinographic findings after intravitreal injection of acyclovir in rabbits
Abstract
Objectives: Acute retinal necrosis is a rapidly progressive and devastating viral retinitis caused by the herpesvirus family. Systemic acyclovir is the treatment of choice; however, the progression of retinal lesions ceases approximately 2 days after treatment initiation. An intravitreal injection of acyclovir may be used an adjuvant therapy during the first 2 days of treatment when systemically administered acyclovir has not reached therapeutic levels in the retina. The aims of this study were to determine the pharmacokinetic profile of acyclovir in the rabbit vitreous after intravitreal injection and the functional effects of acyclovir in the rabbit retina.
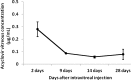
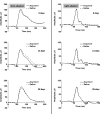
Methods: Acyclovir (Acyclovir; Bedford Laboratories, Bedford, OH, USA) 1 mg in 0.1 mL was injected into the right eye vitreous of 32 New Zealand white rabbits, and 0.1 mL sterile saline solution was injected into the left eye as a control. The animals were sacrificed after 2, 9, 14, or 28 days. The eyes were enucleated, and the vitreous was removed. The half-life of acyclovir was determined using high-performance liquid chromatography. Electroretinograms were recorded on days 2, 9, 14, and 28 in the eight animals that were sacrificed 28 days after injection according to a modified protocol of the International Society for Clinical Electrophysiology of Vision.
Results: Acyclovir rapidly decayed in the vitreous within the first two days after treatment and remained at low levels from day 9 onward. The eyes that were injected with acyclovir did not present any electroretinographic changes compared with the control eyes.
Conclusions: The vitreous half-life of acyclovir is short, and the electrophysiological findings suggest that the intravitreal delivery of 1 mg acyclovir is safe and well tolerated by the rabbit retina.
Figures

Similar articles
-
Evaluation of vitreous clearance and potential retinal toxicity of intravitreal lornoxicam (xefo).J Ocul Pharmacol Ther. 2013 Sep;29(7):627-32. doi: 10.1089/jop.2012.0194. Epub 2013 Apr 4. J Ocul Pharmacol Ther. 2013. PMID: 23556534
-
Ocular toxicity of intravitreal clarithromycin.Retina. 1999;19(5):442-6. doi: 10.1097/00006982-199909000-00013. Retina. 1999. PMID: 10546942
-
Ocular and systemic toxicity of intravitreal topotecan in rabbits for potential treatment of retinoblastoma.Exp Eye Res. 2013 Mar;108:103-9. doi: 10.1016/j.exer.2013.01.002. Epub 2013 Jan 16. Exp Eye Res. 2013. PMID: 23333535
-
Pharmacokinetics, electrophysiological, and morphological effects of the intravitreal injection of mycophenolic acid in rabbits.J Ocul Pharmacol Ther. 2014 Aug;30(6):502-11. doi: 10.1089/jop.2013.0236. Epub 2014 May 14. J Ocul Pharmacol Ther. 2014. PMID: 24828287 Free PMC article.
-
Intraocular toxicity and pharmacokinetics of candesartan in a rabbit model.Invest Ophthalmol Vis Sci. 2011 May 2;52(6):2924-9. doi: 10.1167/iovs.10-5850. Invest Ophthalmol Vis Sci. 2011. PMID: 21228384
Cited by
-
Test-retest reliability of scotopic full-field electroretinograms in rabbits.Doc Ophthalmol. 2017 Jun;134(3):157-165. doi: 10.1007/s10633-017-9582-1. Epub 2017 Mar 16. Doc Ophthalmol. 2017. PMID: 28303363
-
Corosolic acid: antiangiogenic activity and safety of intravitreal injection in rats eyes.Doc Ophthalmol. 2019 Jun;138(3):181-194. doi: 10.1007/s10633-019-09682-x. Epub 2019 Feb 26. Doc Ophthalmol. 2019. PMID: 30809742
-
Comparison between albino and pigmented rabbit ERGs.Doc Ophthalmol. 2018 Apr;136(2):113-123. doi: 10.1007/s10633-018-9628-z. Epub 2018 Mar 23. Doc Ophthalmol. 2018. PMID: 29572760
-
Toxicity and in vivo release profile of sirolimus from implants into the vitreous of rabbits' eyes.Doc Ophthalmol. 2019 Feb;138(1):3-19. doi: 10.1007/s10633-018-9664-8. Epub 2018 Nov 19. Doc Ophthalmol. 2019. PMID: 30456454
-
Intravitreal injection of the synthetic peptide LyeTx I b, derived from a spider toxin, into the rabbit eye is safe and prevents neovascularization in a chorio-allantoic membrane model.J Venom Anim Toxins Incl Trop Dis. 2018 Nov 21;24:31. doi: 10.1186/s40409-018-0168-5. eCollection 2018. J Venom Anim Toxins Incl Trop Dis. 2018. PMID: 30479614 Free PMC article.
References
-
- Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300. - PubMed
-
- Fisher JP, Lewis ML, Blumenkranz M, Culbertson WW, Flynn HW, Jr, Clarkson JG, et al. The acute retinal necrosis syndrome. Part 1: Clinical manifestations. Ophthalmology. 1982;89(12):1309–16. - PubMed
-
- Crapotta JA, Freeman WR, Feldman RM, Lowder CY, Ambler JS, Parker CE, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13(3):208–13. - PubMed
-
- Schaeffer HJ, Beauchamp L, de Miranda P, Elion GB, Bauer DJ, Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272(5654):583–5. - PubMed
-
- Rosenberry KR, Bryan CK, Sohn CA. Acyclovir: evaluation of a new antiviral agent. Clin Pharm. 1982;1(5):399–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

