EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols
- PMID: 22948490
- PMCID: PMC3437409
- DOI: 10.1038/leu.2012.122
EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols
Abstract
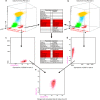
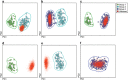
The EU-supported EuroFlow Consortium aimed at innovation and standardization of immunophenotyping for diagnosis and classification of hematological malignancies by introducing 8-color flow cytometry with fully standardized laboratory procedures and antibody panels in order to achieve maximally comparable results among different laboratories. This required the selection of optimal combinations of compatible fluorochromes and the design and evaluation of adequate standard operating procedures (SOPs) for instrument setup, fluorescence compensation and sample preparation. Additionally, we developed software tools for the evaluation of individual antibody reagents and antibody panels. Each section describes what has been evaluated experimentally versus adopted based on existing data and experience. Multicentric evaluation demonstrated high levels of reproducibility based on strict implementation of the EuroFlow SOPs and antibody panels. Overall, the 6 years of extensive collaborative experiments and the analysis of hundreds of cell samples of patients and healthy controls in the EuroFlow centers have provided for the first time laboratory protocols and software tools for fully standardized 8-color flow cytometric immunophenotyping of normal and malignant leukocytes in bone marrow and blood; this has yielded highly comparable data sets, which can be integrated in a single database.
Figures
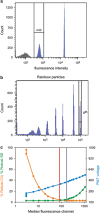

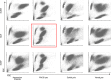




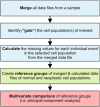
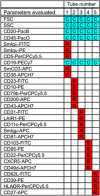


References
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues4th edn.International Agency for Research on Cancer: Lyon; 2008. 439 pp.
-
- Davis BH, Holden JT, Bene MC, Borowitz MJ, Braylan RC, Cornfield D, et al. Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: medical indications. Cytometry B Clin Cytom. 2007;72 (Suppl 1:S5–S13. - PubMed
-
- Wood BL, Arroz M, Barnett D, DiGiuseppe J, Greig B, Kussick SJ, et al. Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry B Clin Cytom. 2007;72 (Suppl 1:S14–S22. - PubMed
-
- Stewart CC, Behm FG, Carey JL, Cornbleet J, Duque RE, Hudnall SD, et al. U.S.-Canadian Consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: selection of antibody combinations. Cytometry. 1997;30:231–235. - PubMed
-
- Stetler-Stevenson M, Ahmad E, Barnett D, Braylan R, DiGiuseppe J, Marti G, et al. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells. Approved guideline. 2nd edn. CLSI document H43-A2 ed. Clinical and Laboratory Standards Institute: Wayne, PA, 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

