The amniote paratympanic organ develops from a previously undiscovered sensory placode
- PMID: 22948823
- PMCID: PMC3518548
- DOI: 10.1038/ncomms2036
The amniote paratympanic organ develops from a previously undiscovered sensory placode
Abstract
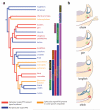
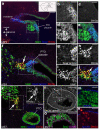
The paratympanic organ, a mechanosensory hair cell-containing pouch in the amniote middle ear, was first described 100 years ago, yet its origins remain unresolved. Homology with the anamniote spiracular organ is supported by association with homologous skeletal elements and similar central targets of afferent neurons, suggesting it might be a remnant of the water-dependent lateral line system, otherwise lost during the amniote transition to terrestrial life. However, this is incompatible with studies suggesting that it arises from the first epibranchial (geniculate) placode. Here we show that a previously undiscovered Sox2-positive placode, immediately dorsal to the geniculate placode, forms the paratympanic organ and its afferent neurons, which are molecularly and morphologically distinct from geniculate neurons. These data remove the only obstacle to accepting the homology of the paratympanic organ and spiracular organ. We hypothesize that the paratympanic organ/spiracular organ represents an ancient head ectoderm module, developmentally and evolutionarily independent of both lateral line and epibranchial placodes.
Figures






References
-
- Baker CVH, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 2001;232:1–61. - PubMed
-
- Schlosser G. Induction and specification of cranial placodes. Dev. Biol. 2006;294:303–51. - PubMed
-
- Schlosser G. Making senses: development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 2010;283:129–234. - PubMed
-
- von Bartheld CS. Functional morphology of the paratympanic organ in the middle ear of birds. Brain Behav. Evol. 1994;44:61–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

