Targeting the substrate preference of a type I nitroreductase to develop antitrypanosomal quinone-based prodrugs
- PMID: 22948871
- PMCID: PMC3486551
- DOI: 10.1128/AAC.01227-12
Targeting the substrate preference of a type I nitroreductase to develop antitrypanosomal quinone-based prodrugs
Abstract
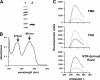
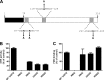
Nitroheterocyclic prodrugs are used to treat infections caused by Trypanosoma cruzi and Trypanosoma brucei. A key component in selectivity involves a specific activation step mediated by a protein homologous with type I nitroreductases, enzymes found predominantly in prokaryotes. Using data from determinations based on flavin cofactor, oxygen-insensitive activity, substrate range, and inhibition profiles, we demonstrate that NTRs from T. cruzi and T. brucei display many characteristics of their bacterial counterparts. Intriguingly, both enzymes preferentially use NADH and quinones as the electron donor and acceptor, respectively, suggesting that they may function as NADH:ubiquinone oxidoreductases in the parasite mitochondrion. We exploited this preference to determine the trypanocidal activity of a library of aziridinyl benzoquinones against bloodstream-form T. brucei. Biochemical screens using recombinant NTR demonstrated that several quinones were effective substrates for the parasite enzyme, having K(cat)/K(m) values 2 orders of magnitude greater than those of nifurtimox and benznidazole. In tests against T. brucei, antiparasitic activity mirrored the biochemical data, with the most potent compounds generally being preferred enzyme substrates. Trypanocidal activity was shown to be NTR dependent, as parasites with elevated levels of this enzyme were hypersensitive to the aziridinyl agent. By unraveling the biochemical characteristics exhibited by the trypanosomal NTRs, we have shown that quinone-based compounds represent a class of trypanocidal compound.
Figures



References
-
- Armstrong JM. 1964. The molar extinction coefficient of 2,6-dichlorophenol indophenol. Biochim. Biophy. Acta 86:194–197 - PubMed
-
- Begleiter A. 2000. Clinical applications of quinone-containing alkylating agents. Front. Biosci. 5:E153–E171 - PubMed
-
- Bern C, Montgomery SP. 2009. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 49:e52–e54 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

