Structure-activity relationships among the kanamycin aminoglycosides: role of ring I hydroxyl and amino groups
- PMID: 22948879
- PMCID: PMC3497201
- DOI: 10.1128/AAC.01326-12
Structure-activity relationships among the kanamycin aminoglycosides: role of ring I hydroxyl and amino groups
Abstract
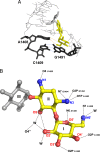
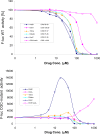
The kanamycins form an important subgroup of the 4,6-disubstituted 2-deoxystreptamine aminoglycoside antibiotics, comprising kanamycin A, kanamycin B, tobramycin, and dibekacin. These compounds interfere with protein synthesis by targeting the ribosomal decoding A site, and they differ in the numbers and locations of amino and hydroxy groups of the glucopyranosyl moiety (ring I). We synthesized kanamycin analogues characterized by subtle variations of the 2' and 6' substituents of ring I. The functional activities of the kanamycins and the synthesized analogues were investigated (i) in cell-free translation assays on wild-type and mutant bacterial ribosomes to study drug-target interaction, (ii) in MIC assays to assess antibacterial activity, and (iii) in rabbit reticulocyte translation assays to determine activity on eukaryotic ribosomes. Position 2' forms an intramolecular H bond with O5 of ring II, helping the relative orientations of the two rings with respect to each other. This bond becomes critical for drug activity when a 6'-OH substituent is present.
Figures
References
-
- Bastida A, et al. 2006. Exploring the use of conformationally locked aminoglycosides as a new strategy to overcome bacterial resistance. J. Am. Chem. Soc. 128:100–116 - PubMed
-
- Blount KF, Zhao F, Hermann T, Tor Y. 2005. Conformational constraint as a means for understanding RNA-aminoglycoside specificity. J. Am. Chem. Soc. 127:9818–9829 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

