Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis
- PMID: 22949039
- PMCID: PMC3638725
- DOI: 10.1002/stem.1218
Immunomodulation by transplanted human embryonic stem cell-derived oligodendroglial progenitors in experimental autoimmune encephalomyelitis
Abstract
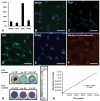
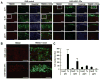
Transplantation of embryonic stem cells and their neural derivatives can lead to amelioration of the disease symptoms of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS). Oligodendroglial progenitors (OPs), derived from human embryonic stem cells (hESC, HES-1), were labeled with superparamagnetic iron oxide and transduced with luciferase. At 7 days following induction of EAE in C57/BL6 mice, 1 × 10(6) cells were transplanted in the ventricles of C57/BL6 mice and noninvasively monitored by magnetic resonance and bioluminescence imaging. Cells were found to remain within the cerebroventricular system and did not survive for more than 10 days. However, EAE mice that received hESC-OPs showed a significant improvement in neurological disability scores (0.9 ± 0.2; n = 12) compared to that of control animals (3.3 ± 0.4; n = 12) at day 15 post-transplantation. Histopathologically, transplanted hESC-OPs generated TREM2-positive CD45 cells, increased TIMP-1 expression, confined inflammatory cells within the subarachnoid space, and gave rise to higher numbers of Foxp3-positive regulatory T cells in the spinal cord and spleen. Our results suggest that transplantation of hESC-OPs can alter the pathogenesis of EAE through immunomodulation, potentially providing new avenues for stem cell-based treatment of MS.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
The authors indicate no potential conflict of interest.
Disclosure of potential conflicts of interest is found at the end of this article.
Figures
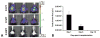
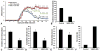



References
-
- Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. - PubMed
-
- Hohlfeld R, Wekerle H. Drug insight: Using monoclonal antibodies to treat multiple sclerosis. Nat Clin Pract Neurol. 2005;1:34–44. - PubMed
-
- Pluchino S, Zanotti L, Brini E, et al. Regeneration and repair in multiple sclerosis: The role of cell transplantation. Neurosci Lett. 2009;456:101–106. - PubMed
-
- Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

