Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial
- PMID: 22949154
- PMCID: PMC4979199
- DOI: 10.1200/JCO.2011.38.7522
Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial
Abstract
Purpose: Bosutinib is an oral Src/Abl tyrosine kinase inhibitor. The phase III Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia (BELA) trial compared bosutinib with imatinib in newly diagnosed, chronic-phase chronic myeloid leukemia (CML).
Patients and methods: A total of 502 patients were randomly assigned 1:1 to bosutinib 500 mg per day or imatinib 400 mg per day.
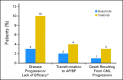
Results: The complete cytogenetic response (CCyR) rate at 12 months was not different for bosutinib (70%; 95% CI, 64% to 76%) versus imatinib (68%; 95% CI, 62% to 74%; two-sided P = .601); therefore, the study did not achieve its primary end point. The major molecular response (MMR) rate at 12 months was higher with bosutinib (41%; 95% CI, 35% to 47%) compared with imatinib (27%; 95% CI, 22% to 33%; two-sided P < .001). Time to CCyR and MMR was faster with bosutinib compared with imatinib (two-sided P < .001 for both). On-treatment transformation to accelerated/blast phase occurred in four patients (2%) on bosutinib compared with 10 patients (4%) on imatinib. A total of three CML-related deaths occurred on the bosutinib arm compared with eight on the imatinib arm. The safety profiles of bosutinib and imatinib were distinct; GI and liver-related events were more frequent with bosutinib, whereas neutropenia, musculoskeletal disorders, and edema were more frequent with imatinib.
Conclusion: This ongoing trial did not meet its primary end point of CCyR at 12 months, despite the observed higher MMR rate at 12 months, faster times to CCyR and MMR, fewer on-treatment transformations to accelerated/blast phase, and fewer CML-related deaths with bosutinib compared with imatinib. Each drug had a distinct safety profile.
Trial registration: ClinicalTrials.gov NCT00574873.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: Chronic myelogenous leukemia—Version 2.2011. http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. - PubMed
-
- Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. - PubMed
-
- O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. - PubMed
-
- Hehlmann R, Lauseker M, Jung-Munkwitz S, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. 2011;29:1634–1642. - PubMed
-
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

