Oxidative stress responses involve oxidation of a conserved ubiquitin pathway enzyme
- PMID: 22949505
- PMCID: PMC3486136
- DOI: 10.1128/MCB.00559-12
Oxidative stress responses involve oxidation of a conserved ubiquitin pathway enzyme
Abstract
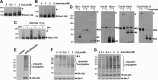
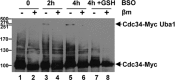
Although it is vital that cells detect and respond to oxidative stress to allow adaptation and repair damage, the underlying sensing and signaling mechanisms that control these responses are unclear. Protein ubiquitinylation plays an important role in controlling many biological processes, including cell division. In Saccharomyces cerevisiae, ubiquitinylation involves a single E1 enzyme, Uba1, with multiple E2s and E3s providing substrate specificity. For instance, the conserved E2 Cdc34 ubiquitinylates many substrates, including the cyclin-dependent kinase inhibitor Sic1, targeting it for degradation to allow cell cycle progression. Here we reveal that, in contrast to other ubiquitin pathway E2 enzymes, Cdc34 is particularly sensitive to oxidative inactivation, through sequestration of the catalytic cysteine in a disulfide complex with Uba1, by levels of oxidant that do not reduce global ubiquitinylation of proteins. This Cdc34 oxidation is associated with (i) reduced levels of Cdc34-ubiquitin thioester forms, (ii) increased stability of at least one Cdc34 substrate, Sic1, and (iii) Sic1-dependent delay in cell cycle progression. Together, these data reveal that the differential sensitivity of a ubiquitin pathway E2 enzyme to oxidation is utilized as a stress-sensing mechanism to respond to oxidative stress.
Figures






References
-
- Banerjee A, Gregori L, Xu Y, Chau V. 1993. The bacterially expressed yeast CDC34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. J. Biol. Chem. 268: 5668–5675 - PubMed
-
- Beckman KB, Ames BN. 1997. Oxidative decay of DNA. J. Biol. Chem. 272: 19633–19636 - PubMed
-
- Berben G, Dumont J, Gilliquet V, Bolle PA, Hilger F. 1991. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast 7: 475–477 - PubMed
-
- Berlett BS, Stadtman ER. 1997. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272: 20313–20316 - PubMed
-
- Bossis G, Melchior F. 2006. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol. Cell 21: 349–357 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
