Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study
- PMID: 22949583
- PMCID: PMC3611995
- DOI: 10.1093/ntr/nts173
Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study
Abstract
Introduction: There is increasing evidence that response to pharmacological treatment for nicotine dependence may be moderated by genetic polymorphisms. However, the feasibility, acceptability, and impact of genetically tailoring treatment in real-world clinical settings are unknown.
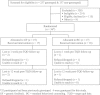
Methods: We conducted a multiphased, mixed-methods feasibility study with current smokers to develop and evaluate a patient-centered, theoretically grounded personalized medicine treatment protocol. The initial research phase included formative work to develop intervention materials. The second phase included a randomized pilot trial to evaluate the intervention. Trial participants (n = 36) were genotyped for ANKK1 rs1800497 and were randomized to receive genetic feedback (GF) plus standard behavioral counseling (BC) for smoking cessation or BC without GF. All participants received genetically tailored pharmacotherapy (nicotine patch or bupropion).
Results: The intervention was feasible to implement and was acceptable to participants based on satisfaction ratings and objective measures of participation. There was no evidence that the GF resulted in adverse psychological outcomes (e.g., depression, fatalism, reduced perceived control over quitting, differential motivation for quitting) based on quantitative or qualitative outcomes.
Conclusions: Study results suggest that it is feasible to offer treatment within a health care setting that includes genetically tailored pharmacotherapy and doing so had no apparent adverse psychological impacts. Further evaluation of pharmacogenetically tailored smoking cessation interventions appears warranted.
References
-
- Allison M. (2008). Is personalized medicine finally arriving? Nature Biotechnology 26 509–517doi:10.1038/nbt0508–509 - PubMed
-
- Altman R. B. (2011). Pharmacogenomics: “Noninferiority” is sufficient for initial implementation Clinical Pharmacology and Therapeutics 89 348–350doi:10.1038/clpt.2010.310 - PubMed
-
- Baars M. J., Henneman L., Ten Kate L. P. (2005). Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: A global problem Genetics in Medicine 7 605–610doi:0.1097/01.gim.0000182895.28432.c7 - PubMed
-
- Breitling L. P., Müller H., Illig T., Rujescu D., Winterer G., Dahmen N, Brenner H., et al. (2011). Dopamine-related genes and spontaneous smoking cessation in ever-heavy smokers Pharmacogenomics 12 1099–1106 doi:10.2217/pgs.11.74 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical