An inwardly rectifying K+ channel is required for patterning
- PMID: 22949619
- PMCID: PMC3436115
- DOI: 10.1242/dev.078592
An inwardly rectifying K+ channel is required for patterning
Abstract
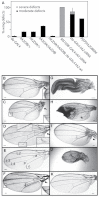
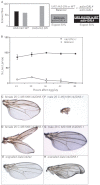
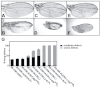
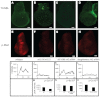
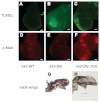
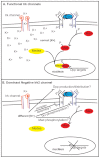
Mutations that disrupt function of the human inwardly rectifying potassium channel KIR2.1 are associated with the craniofacial and digital defects of Andersen-Tawil Syndrome, but the contribution of Kir channels to development is undefined. Deletion of mouse Kir2.1 also causes cleft palate and digital defects. These defects are strikingly similar to phenotypes that result from disrupted TGFβ/BMP signaling. We use Drosophila melanogaster to show that a Kir2.1 homolog, Irk2, affects development by disrupting BMP signaling. Phenotypes of irk2 deficient lines, a mutant irk2 allele, irk2 siRNA and expression of a dominant-negative Irk2 subunit (Irk2DN) all demonstrate that Irk2 function is necessary for development of the adult wing. Compromised Irk2 function causes wing-patterning defects similar to those found when signaling through a Drosophila BMP homolog, Decapentaplegic (Dpp), is disrupted. To determine whether Irk2 plays a role in the Dpp pathway, we generated flies in which both Irk2 and Dpp functions are reduced. Irk2DN phenotypes are enhanced by decreased Dpp signaling. In wild-type flies, Dpp signaling can be detected in stripes along the anterior/posterior boundary of the larval imaginal wing disc. Reducing function of Irk2 with siRNA, an irk2 deletion, or expression of Irk2DN reduces the Dpp signal in the wing disc. As Irk channels contribute to Dpp signaling in flies, a similar role for Kir2.1 in BMP signaling may explain the morphological defects of Andersen-Tawil Syndrome and the Kir2.1 knockout mouse.
Figures



References
-
- Adachi-Yamada T., O’Connor M. B. (2002). Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev. Biol. 251, 74–90 - PubMed
-
- Adachi-Yamada T., Fujimura-Kamada K., Nishida Y., Matsumoto K. (1999). Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature 400, 166–169 - PubMed
-
- Andersen E. D., Krasilnikoff P. A., Overvad H. (1971). Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatr. Scand. 60, 559–564 - PubMed
-
- Bendahhou S., Donaldson M. R., Plaster N. M., Tristani-Firouzi M., Fu Y. H., Ptácek L. J. (2003). Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J. Biol. Chem. 278, 51779–51785 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

