Sex, prions, and plasmids in yeast
- PMID: 22949655
- PMCID: PMC3479589
- DOI: 10.1073/pnas.1213449109
Sex, prions, and plasmids in yeast
Abstract
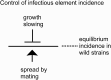
Even deadly prions may be widespread in nature if they spread by infection faster than they kill off their hosts. The yeast prions [PSI+] and [URE3] (amyloids of Sup35p and Ure2p) were not found in 70 wild strains, while [PIN+] (amyloid of Rnq1p) was found in ∼16% of the same population. Yeast prion infection occurs only by mating, balancing the detrimental effects of carrying the prion. We estimated the frequency of outcross mating as about 1% of mitotic doublings from the known detriment of carrying the 2-μm DNA plasmid (∼1%) and its frequency in wild populations (38/70). We also estimated the fraction of total matings that are outcross matings (∼23-46%) from the fraction of heterozygosity at the highly polymorphic RNQ1 locus (∼46%). These results show that the detriment of carrying even the mildest forms of [PSI+], [URE3], or [PIN+] is greater than 1%. We find that Rnq1p polymorphisms in wild strains include several premature stop codon alleles that cannot propagate [PIN+] from the reference allele and others with several small deletions and point mutations which show a small transmission barrier. Wild strains carrying [PIN+] are far more likely to be heterozygous at RNQ1 and other loci than are [pin-] strains, probably reflecting its being a sexually transmitted disease. Because sequence differences are known to block prion propagation or ameliorate its pathogenic effects, we hypothesize that polymorphism of RNQ1 was selected to protect cells from detrimental effects of the [PIN+] prion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
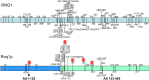
 indicates that the polymorphism resulted in a premature stop codon.
indicates that the polymorphism resulted in a premature stop codon.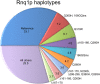
References
-
- Aguzzi A, Baumann F, Bremer J. The prion’s elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. - PubMed
-
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. - PubMed
-
- Wickner RB. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

