Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain
- PMID: 22949658
- PMCID: PMC3465435
- DOI: 10.1073/pnas.1203534109
Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain
Abstract
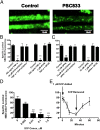
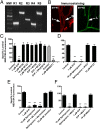
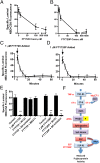
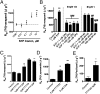
P-glycoprotein, an ATP-driven drug efflux pump, is a major obstacle to the delivery of small-molecule drugs across the blood-brain barrier and into the CNS. Here we test a unique signaling-based strategy to overcome this obstacle. We used a confocal microscopy-based assay with isolated rat brain capillaries to map a signaling pathway that within minutes abolishes P-glycoprotein transport activity without altering transporter protein expression or tight junction permeability. This pathway encompasses elements of proinflammatory- (TNF-α) and sphingolipid-based signaling. Critical to this pathway was signaling through sphingosine-1-phosphate receptor 1 (S1PR1). In brain capillaries, S1P acted through S1PR1 to rapidly and reversibly reduce P-glycoprotein transport activity. Sphingosine reduced transport by a sphingosine kinase-dependent mechanism. Importantly, fingolimod (FTY720), a S1P analog recently approved for treatment of multiple sclerosis, also rapidly reduced P-glycoprotein activity; similar effects were found with the active, phosphorylated metabolite (FTY720P). We validated these findings in vivo using in situ brain perfusion in rats. Administration of S1P, FTY720, or FTY729P increased brain uptake of three radiolabeled P-glycoprotein substrates, (3)H-verapamil (threefold increase), (3)H-loperamide (fivefold increase), and (3)H-paclitaxel (fivefold increase); blocking S1PR1 abolished this effect. Tight junctional permeability, measured as brain (14)C-sucrose accumulation, was not altered. Therefore, targeting signaling through S1PR1 at the blood-brain barrier with the sphingolipid-based drugs, FTY720 or FTY720P, can rapidly and reversibly reduce basal P-glycoprotein activity and thus improve delivery of small-molecule therapeutics to the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Ferry DR, Traunecker H, Kerr DJ. Clinical trials of P-glycoprotein reversal in solid tumours. Eur J Cancer. 1996;32A:1070–1081. - PubMed
-
- Krishna R, St-Louis M, Mayer LD. Increased intracellular drug accumulation and complete chemosensitization achieved in multidrug-resistant solid tumors by co-administering valspodar (PSC 833) with sterically stabilized liposomal doxorubicin. Int J Cancer. 2000;85:131–141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

