Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover
- PMID: 22949660
- PMCID: PMC3458384
- DOI: 10.1073/pnas.1205037109
Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover
Abstract
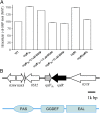
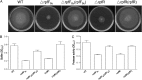
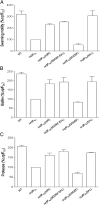
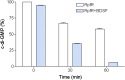
Many bacterial pathogens produce diffusible signal factor (DSF)-type quorum sensing (QS) signals in modulation of virulence and biofilm formation. Previous work on Xanthomonas campestris showed that the RpfC/RpfG two-component system is involved in sensing and responding to DSF signals, but little is known in other microorganisms. Here we show that in Burkholderia cenocepacia the DSF-family signal cis-2-dodecenoic acid (BDSF) negatively controls the intracellular cyclic dimeric guanosine monophosphate (c-di-GMP) level through a receptor protein RpfR, which contains Per/Arnt/Sim (PAS)-GGDEF-EAL domains. RpfR regulates the same phenotypes as BDSF including swarming motility, biofilm formation, and virulence. In addition, the BDSF(-) mutant phenotypes could be rescued by in trans expression of RpfR, or its EAL domain that functions as a c-di-GMP phosphodiesterase. BDSF is shown to bind to the PAS domain of RpfR with high affinity and stimulates its phosphodiesterase activity through induction of allosteric conformational changes. Our work presents a unique and widely conserved DSF-family signal receptor that directly links the signal perception to c-di-GMP turnover in regulation of bacterial physiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fuqua C, Greenberg EP. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. - PubMed
-
- Zhang LH, Dong YH. Quorum sensing and signal interference: Diverse implications. Mol Microbiol. 2004;53:1563–1571. - PubMed
-
- Deng Y, Wu J, Tao F, Zhang LH. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev. 2011;111:160–173. - PubMed
-
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. - PubMed
-
- Williams P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology. 2007;153:3923–3938. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

