An "aprotic" Tamao oxidation/syn-selective tautomerization reaction for the efficient synthesis of the C1-C9 fragment of fludelone
- PMID: 22950417
- PMCID: PMC3466590
- DOI: 10.1021/ol302221s
An "aprotic" Tamao oxidation/syn-selective tautomerization reaction for the efficient synthesis of the C1-C9 fragment of fludelone
Abstract
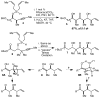
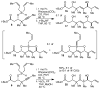
An efficient synthesis of the C(1)-C(9) fragment of fludelone has been developed. The key step is a tandem silylformylation-crotylsilylation/Tamao oxidation sequence that establishes the C(5) ketone, the C(6), C(7), and C(8) stereocenters, and the C(9) alkene in a single operation from a readily accessed starting material. The stereochemical outcome at C(6) depends critically on the development of an "aprotic" Tamao oxidation, which leads to a reversal in the intrinsic diastereoselectivity observed using "standard" Tamao oxidation conditions.
Figures



Similar articles
-
A Highly Stereoselective, Efficient, and Scalable Synthesis of the C(1)-C(9) Fragment of the Epothilones.Org Lett. 2015 Dec 4;17(23):5858-61. doi: 10.1021/acs.orglett.5b03034. Epub 2015 Nov 12. Org Lett. 2015. PMID: 26561788 Free PMC article.
-
Beyond the Roche ester: a new approach to polypropionate stereotriad synthesis.Org Lett. 2014 Feb 21;16(4):1180-3. doi: 10.1021/ol500051e. Epub 2014 Feb 6. Org Lett. 2014. PMID: 24502345 Free PMC article.
-
Tandem silylformylation-crotylsilylation/Tamao oxidation of internal alkynes: a remarkable example of generating complexity from simplicity.Org Lett. 2008 Dec 18;10(24):5593-6. doi: 10.1021/ol802489w. Org Lett. 2008. PMID: 19007175 Free PMC article.
-
Application of ring-closing metathesis reactions in the synthesis of epothilones.J Nat Prod. 2004 Feb;67(2):139-43. doi: 10.1021/np030540k. J Nat Prod. 2004. PMID: 14987048 Review.
-
On the remarkable antitumor properties of fludelone: how we got there.Angew Chem Int Ed Engl. 2005 May 6;44(19):2838-50. doi: 10.1002/anie.200461751. Angew Chem Int Ed Engl. 2005. PMID: 15880547 Review.
Cited by
-
A Highly Stereoselective, Efficient, and Scalable Synthesis of the C(1)-C(9) Fragment of the Epothilones.Org Lett. 2015 Dec 4;17(23):5858-61. doi: 10.1021/acs.orglett.5b03034. Epub 2015 Nov 12. Org Lett. 2015. PMID: 26561788 Free PMC article.
-
Tailoring chemoenzymatic oxidation via in situ peracids.Org Biomol Chem. 2019 Nov 6;17(43):9418-9424. doi: 10.1039/c9ob01814j. Org Biomol Chem. 2019. PMID: 31650153 Free PMC article.
-
Stereoselective one-pot synthesis of polypropionates.Nat Commun. 2017 Sep 25;8(1):679. doi: 10.1038/s41467-017-00787-y. Nat Commun. 2017. PMID: 28947767 Free PMC article.
-
Beyond the Roche ester: a new approach to polypropionate stereotriad synthesis.Org Lett. 2014 Feb 21;16(4):1180-3. doi: 10.1021/ol500051e. Epub 2014 Feb 6. Org Lett. 2014. PMID: 24502345 Free PMC article.
-
A highly step-economical synthesis of dictyostatin.Angew Chem Int Ed Engl. 2013 Jun 24;52(26):6757-61. doi: 10.1002/anie.201302565. Epub 2013 May 10. Angew Chem Int Ed Engl. 2013. PMID: 23666786 Free PMC article. No abstract available.
References
-
- Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. ChemMedChem. 2007;2:396. - PubMed
- Altmann KH, Höfle G, Müller R, Mulzer J, Prantz K. Progress in the Chemistry of Organic Natural Products. In: Kinghorn AD, Falk H, Kobayashi J, editors. The Epothilones: An Outstanding Family of Anti-Tumor Agents. From Soil to the Clinic. Vol. 90. Springer; Vienna: 2009. p. 260.
-
-
For a recent review and lead references, see: Watkins EB, Chittiboyina AG, Avery MA. Eur J Org Chem. 2006:4071.
-
-
- Zacuto MJ, Leighton JL. J Am Chem Soc. 2000;122:8587.
- O’Malley SJ, Leighton JL. Angew Chem Int Ed. 2001;40:2915. - PubMed
- Zacuto MJ, O’Malley SJ, Leighton JL. J Am Chem Soc. 2002;124:7890. - PubMed
- Wang X, Meng Q, Nation AJ, Leighton JL. J Am Chem Soc. 2002;124:10672. - PubMed
- Schmidt DR, O’Malley SJ, Leighton JL. J Am Chem Soc. 2003;125:1190. - PubMed
- Zacuto MJ, O’Malley SJ, Leighton JL. Tetrahedron. 2003;59:8889.
- Schmidt DR, Park PK, Leighton JL. Org Lett. 2003;5:3535. - PubMed
- Wang X, Meng Q, Perl NR, Xu Y, Leighton JL. J Am Chem Soc. 2005;127:12806. - PubMed
- Spletstoser JT, Zacuto MJ, Leighton JL. Org Lett. 2008;10:5593. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

