The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor
- PMID: 22950459
- PMCID: PMC3488569
- DOI: 10.1186/1742-2094-9-211
The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor
Abstract
Background: Post-ischemic microglial activation may contribute to neuronal damage through the release of large amounts of pro-inflammatory cytokines and neurotoxic factors. The involvement of microRNAs (miRNAs) in the pathogenesis of disorders related to the brain and central nervous system has been previously studied, but it remains unknown whether the production of pro-inflammatory cytokines is regulated by miRNAs.
Methods: BV-2 and primary rat microglial cells were activated by exposure to oxygen-glucose deprivation (OGD). Global cerebral ischemia was induced using the four-vessel occlusion (4-VO) model in rats. Induction of pro-inflammatory and neurotoxic factors, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and nitric oxide (NO), were assessed by ELISA, immunofluorescence, and the Griess assay, respectively. The miRNA expression profiles of OGD-activated BV-2 cells were subsequently compared with the profiles of resting cells in a miRNA microarray. BV-2 and primary rat microglial cells were transfected with miR-181c to evaluate its effects on TNF-α production after OGD. In addition, a luciferase reporter assay was conducted to confirm whether TNF-α is a direct target of miR-181c.
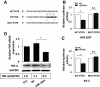
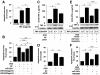
Results: OGD induced BV-2 microglial activation in vitro, as indicated by the overproduction of TNF-α, IL-1β, and NO. Global cerebral ischemia/reperfusion injury induced microglial activation and the release of pro-inflammatory cytokines in the hippocampus. OGD also downregulated miR-181c expression and upregulated TNF-α expression. Overproduction of TNF-α after OGD-induced microglial activation provoked neuronal apoptosis, whereas the ectopic expression of miR-181c partially protected neurons from cell death caused by OGD-activated microglia. RNAinterference-mediated knockdown of TNF-α phenocopied the effect of miR-181c-mediated neuronal protection, whereas overexpression of TNF-α blocked the miR-181c-dependent suppression of apoptosis. Further studies showed that miR-181c could directly target the 3'-untranslated region of TNF-α mRNA, suppressing its mRNA and protein expression.
Conclusions: Our data suggest a potential role for miR-181c in the regulation of TNF-α expression after ischemia/hypoxia and microglia-mediated neuronal injury.
Figures





References
-
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. - DOI - PubMed
-
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. - DOI - PMC - PubMed
-
- Griffin WS, Sheng JG, Gentleman SM, Graham DI, Mrak RE, Roberts GW. Microglial interleukin-1 alpha expression in human head injury: correlations with neuronal and neuritic beta-amyloid precursor protein expression. Neurosci Lett. 1994;176:133–136. doi: 10.1016/0304-3940(94)90066-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

