Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold
- PMID: 22950667
- PMCID: PMC3575307
- DOI: 10.1186/1472-6882-12-147
Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold
Abstract
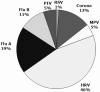
Background: Common cold is caused by a variety of respiratory viruses. The prevalence in children is high, and it potentially contributes to significant morbidity. Iota-carragenan, a polymer derived from red seaweed, has reduced viral load in nasal secretions and alleviated symptoms in adults with common cold.
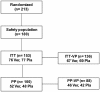
Methods: We have assessed the antiviral and therapeutic activity of a nasal spray containing iota-carrageenan in children with acute symptoms of common cold. A cohort of 153 children between 1-18 years (mean age 5 years), displaying acute symptoms of common cold were randomly assigned to treatment with a nasal spray containing iota-carrageenan (0.12%) as verum or 0.9% sodium chloride solution as placebo for seven days. Symptoms of common cold were recorded and the viral load of respiratory viruses in nasal secretions was determined at two consecutive visits.
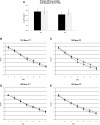
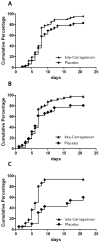
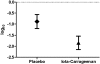
Results: The results of the present study showed no significant difference between the iota carrageenan and the placebo group on the mean of TSS between study days 2-7. Secondary endpoints, such as reduced time to clearance of disease (7.6 vs 9.4 days; p = 0.038), reduction of viral load (p = 0.026), and lower incidence of secondary infections with other respiratory viruses (p = 0.046) indicated beneficial effects of iota-carrageenan in this population. The treatment was safe and well tolerated, with less side effects observed in the verum group compared to placebo.
Conclusion: In this study iota-carrageenan did not alleviate symptoms in children with acute symptoms of common cold, but significantly reduced viral load in nasal secretions that may have important implications for future studies.
Trial registration: ISRCTN52519535, http://www.controlled-trials.com/ISRCTN52519535/
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

