ATP is released from autophagic vesicles to the extracellular space in a VAMP7-dependent manner
- PMID: 22951367
- PMCID: PMC3541285
- DOI: 10.4161/auto.21858
ATP is released from autophagic vesicles to the extracellular space in a VAMP7-dependent manner
Abstract
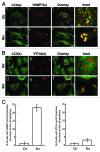
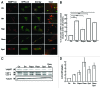
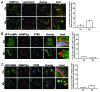
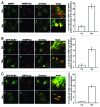
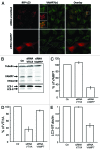
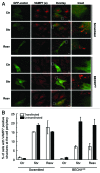
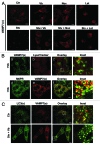
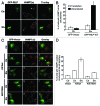
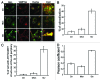
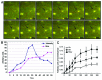
Autophagy is a normal degradative pathway that involves the sequestration of cytoplasmic components and organelles in a vacuole called autophagosome. SNAREs proteins are key molecules of the vesicle fusion machinery. Our results indicate that in a mammalian tumor cell line a subset of VAMP7 (V-SNARE)-positive vacuoles colocalize with LC3 at the cell periphery (focal adhesions) upon starvation. The re-distribution of VAMP7 positive structures is a microtubule-dependent event, with the participation of the motor protein KIF5 and the RAB7 effector RILP. Interestingly, most of the VAMP7-labeled vesicles were loaded with ATP. Moreover, in cells subjected to starvation, these structures fuse with the plasma membrane to release the nucleotide to the extracellular medium. Summarizing, our results show the molecular components involved in the release of ATP to extracellular space, which is recognized as an important autocrine/paracrine signal molecule that participates in the regulation of several cellular functions such as immunogenicity of cancer cell death or inflammation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
