Treatment strategies targeting amyloid β-protein
- PMID: 22951439
- PMCID: PMC3426815
- DOI: 10.1101/cshperspect.a006387
Treatment strategies targeting amyloid β-protein
Abstract
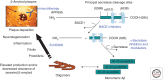
With the advent of the key discovery in the mid-1980s that the amyloid β-protein (Aβ) is the core constituent of the amyloid plaque pathology found in Alzheimer disease (AD), an intensive effort has been underway to attempt to mitigate its role in the hope of treating the disease. This effort fully matured when it was clarified that the Aβ is a normal product of cleavage of the amyloid precursor protein, and well-defined proteases for this process were identified. Further therapeutic options have been developed around the concept of anti-Aβ aggregation inhibitors and the surprising finding that immunization with Aβ itself leads to reduction of pathology in animal models of the disease. Here we review the progress in this field toward the goal of targeting Aβ for treatment and prevention of AD and identify some of the major challenges for the future of this area of medicine.
Figures


References
-
- Acquati F, Accarino M, Nucci C, Fumagalli P, Jovine L, Ottolenghi S, Taramelli R 2000. The gene encoding DRAP (BACE2), a glycosylated transmembrane protein of the aspartic protease family, maps to the down critical region. FEBS Lett 468: 59–64 - PubMed
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ 2003. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: A randomized controlled trial. JAMA 289: 2819–2826 - PubMed
-
- Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D 2006. A Phase II study targeting amyloid-β with 3APS in mild-to-moderate Alzheimer disease. Neurology 67: 1757–1763 - PubMed
-
- Albright CF, Dockens R, Olson RE, Meredith JE, Siemmon R, Lentz KJW, Denton R, Pilcher G, Zaczek R 2008. BMS-708163, a potent and selective γ-secretase inhibitor, decreases CSF Ab at safe and tolerable doses in animals, and humans. In International Conference on Alzheimer’s Disease, 2008, HT-01-05 Alzheimer’s Association, Chicago
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
