Immunogenicity of different stressed IgG monoclonal antibody formulations in immune tolerant transgenic mice
- PMID: 22951518
- PMCID: PMC3502241
- DOI: 10.4161/mabs.22066
Immunogenicity of different stressed IgG monoclonal antibody formulations in immune tolerant transgenic mice
Abstract
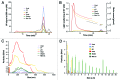
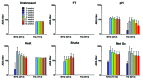
The presence of protein aggregates in biopharmaceutical formulations is of great concern for safety and efficacy reasons. The aim of this study was to correlate the type and amount of IgG monoclonal antibody aggregates with their immunogenic potential. IgG degradation was obtained by freeze-thawing cycles, pH-shift cycles, heating, shaking and metal-catalyzed oxidation. The size, amount, morphology and type of intermolecular bonds of aggregates, as well as structural changes and epitope integrity were characterized. These formulations were injected in mice transgenic (TG) for human genes for Ig heavy and light chains and their non-transgenic (NTG) counterparts. Anti-drug antibody (ADA) titers were determined by bridging ELISA. Both unstressed IgG and freeze-thawed formulation did not induce measurable ADA levels. A mild antibody response was obtained in a fairly small percentage of mice, when injected with shaken, pH-shifted and heated formulations. The metal-catalyzed oxidized IgG formulation was the most immunogenic one, in both ADA titers and number of responders. The overall titers of NTG responders were significantly higher than the ones produced by TG mice, whereas there was no significant difference between the overall number of TG and NTG responders. This study reinforces the important role of protein aggregates on immunogenicity of therapeutic proteins and provides new insight into the immunogenic potential of different types of IgG aggregates. The results indicate that the quality of the IgG aggregates has more impact on the development of an immune response than their quantity or size.
Keywords: IgG; accelerated stress conditions; immunogenicity; protein aggregates; transgenic mice.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
