Toll-like receptor 4 has an essential role in early skin wound healing
- PMID: 22951730
- PMCID: PMC3519973
- DOI: 10.1038/jid.2012.267
Toll-like receptor 4 has an essential role in early skin wound healing
Erratum in
- J Invest Dermatol. 2014 Feb;134(2):583
Abstract
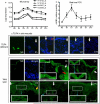
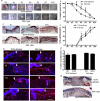
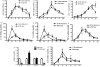
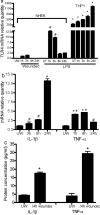
Toll-like receptor 4 (TLR4) has a key role in the initiation of innate immunity and in the regulation of adaptive immune responses. Using microarray analysis and PCR, TLR4 expression was observed to increase in murine skin wounds at the early stages. The cellular location of TLR4 was primarily in keratinocytes at the wound edges. The closure of excisional wounds was significantly delayed in TLR4-deficient (C3H/HeJ) as compared with wild-type mice, and both IL-1β and IL-6 production were significantly lower in the wounds of TLR4-deficient mice. EGF also markedly decreased in the wound edge of epidermis in TLR4-deficient mice. In vitro studies confirmed that a wound stimulus induces TLR4 mRNA expression in primary normal human epidermal keratinocytes (NHEK). In vitro injury also induced the phosphorylation of p38 and JNK MAPK (Jun N-terminal kinase mitogen-activated protein kinase) and the expression of IL-1β and tumor necrosis factor-α by NHEK. Blockade of TLR4 delayed NHEK migration and abolished the phosphorylation of p38 and JNK MAPK, and blockade of TLR4 and/or p38/JNK abolished IL-1β production. The results suggest that inflammatory cytokine production by injured NHEK is stimulated via the TLR4-p38 and JNK MAPK signaling pathway. Together, the results provide evidence for a role of TLR4 at sites of injury, and suggest that TLR4 is an important regulator of wound inflammation.
Figures

References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
-
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. - PubMed
-
- Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. - PubMed
-
- Becker CE, O'Neill LA. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin Immunopathol. 2007;29:239–48. - PubMed
-
- Bettinger DA, Pellicane JV, Tarry WC, Yager DR, Diegelmann RF, Lee R, et al. The role of inflammatory cytokines in wound healing: accelerated healing in endotoxin-resistant mice. J Trauma. 1994;36:810–3. discussion 3-4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

