Persistence of Epstein-Barr virus in self-reactive memory B cells
- PMID: 22951828
- PMCID: PMC3486485
- DOI: 10.1128/JVI.01699-12
Persistence of Epstein-Barr virus in self-reactive memory B cells
Abstract
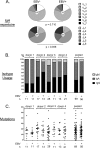
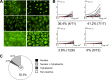
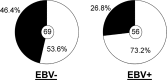
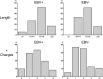
Epstein-Barr virus infection has been epidemiologically associated with the development of multiple autoimmune diseases, particularly systemic lupus erythematosus and multiple sclerosis. Currently, there is no known mechanism that can account for these associations. The germinal-center (GC) model of EBV infection and persistence proposes that EBV gains access to the memory B cell compartment via GC reactions by driving infected cells to differentiate using the virus-encoded LMP1 and LMP2a proteins, which act as functional homologues of CD40 and the B cell receptor, respectively. The ability of LMP2a, when expressed in mice, to allow escape of autoreactive B cells suggests that it could perform a similar role in infected GC B cells, permitting the survival of potentially pathogenic autoreactive B cells. To test this hypothesis, we cloned and expressed antibodies from EBV(+) and EBV(-) memory B cells present during acute infection and profiled their self- and polyreactivity. We find that EBV does persist within self- and polyreactive B cells but find no evidence that it favors the survival of pathogenic autoreactive B cells. On the contrary, EBV(+) memory B cells express lower levels of self-reactive and especially polyreactive antibodies than their uninfected counterparts do. Our work suggests that EBV has only a modest effect on the GC process, which allows it to access and persist within a subtly unique niche of the memory compartment characterized by relatively low levels of self- and polyreactivity. We suggest that this might reflect an active process where EBV and its human host have coevolved so as to minimize the virus's potential to contribute to autoimmune disease.
Figures





References
-
- Alotaibi S, Kennedy J, Tellier R, Stephens D, Banwell B. 2004. Epstein-Barr virus in pediatric multiple sclerosis. JAMA 291:1875–1879 - PubMed
-
- Ascherio A, Munger KL. 2007. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann. Neurol. 61:288–299 - PubMed
-
- Babcock GJ, Hochberg D, Thorley-Lawson AD. 2000. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13:497–506 - PubMed
-
- Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. 1998. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9:405–411 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

