Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic β cell death
- PMID: 22951927
- PMCID: PMC3541286
- DOI: 10.4161/auto.21994
Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic β cell death
Abstract
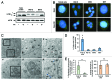
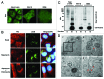
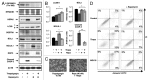
There is a growing evidence of the role of autophagy in pancreatic β cell homeostasis. During development of type 2 diabetes, β cells are required to supply the increased demand of insulin. In such a stage, β cells have to address high ER stress conditions that could lead to abnormal insulin secretion, and ultimately, β cell death and overt diabetes. In this study, we used insulin secretion-deficient β cells derived from fetal mice. These cells present an increased accumulation of polyubiquitinated protein aggregates and LC3B-positive puncta, when compared with insulinoma-derived β cell lines. We found that insulin secretion deficiency renders these cells hypersensitive to endoplasmic reticulum (ER) stress-mediated cell death. Chemical or shRNA-mediated inhibition of autophagy increased β cell death under ER stress. On the other hand, rapamycin treatment increased both autophagy and cell survival under ER stress. Insulin secretion-deficient β cells showed a marked reduction of the antiapoptotic protein BCL2, together with increased BAX expression and ERN1 hyperactivation upon ER stress induction. These results showed how insulin secretion deficiency in β cells may be contributing to ER stress-mediated cell death, and in this regard, we showed how the autophagic response plays a prosurvival role.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
