Activation of the aryl hydrocarbon receptor sensitises human keratinocytes for CD95L- and TRAIL-induced apoptosis
- PMID: 22951985
- PMCID: PMC3461363
- DOI: 10.1038/cddis.2012.127
Activation of the aryl hydrocarbon receptor sensitises human keratinocytes for CD95L- and TRAIL-induced apoptosis
Abstract
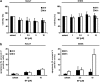
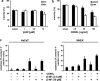
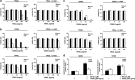
In this study, we have analysed the apoptotic effects of the ubiquitous environmental toxin benzo[a]pyrene (BP) in HaCaT cells and human keratinocytes. Although prolonged exposure to BP was not cytotoxic on its own, a strong enhancement of CD95 (Fas)-mediated apoptosis was observed with BP at concentrations activating the aryl hydrocarbon receptor (AhR). Importantly, the ultimately mutagenic BP-metabolite, that is, (+)-anti-BP-7,8-diol-9,10-epoxide (BPDE), failed to enhance CD95-mediated cell death, suggesting that the observed pro-apoptotic effect of BP is neither associated with DNA adducts nor DNA-damage related signalling. CD95-induced apoptosis was also enhanced by β-naphtoflavone, a well-known agonist of the AhR that does not induce DNA damage, thus suggesting a crucial role for AhR activation. Consistently, BP failed to sensitise for CD95L-induced apoptosis in AhR knockdown HaCaT cells. Furthermore, inhibition of CYP1A1 and/or 1B1 expression did not affect the pro-apoptotic crosstalk. Exposure to BP did not increase expression of CD95, but led to augmented activation of caspase-8. Enhancement of apoptosis was also observed with the TRAIL death receptors that activate caspase-8 and apoptosis by similar mechanisms as CD95. Together, these observations indicate an interference of AhR signalling with the activity of receptor-associated signalling intermediates that are shared by CD95 and TRAIL receptors. Our data thus suggest that AhR agonists can enhance cytokine-mediated adversity upon dermal exposure.
Figures





References
-
- Hall M, Grover PL.Chemical carcinogenesis and mutagenesisIn: Cooper CS, Grover PL, (eds).Handbook of Experimental Pharmacology, Vol ISpringer-Verlag: Berlin; 1990327–372.
-
- Luch A, Baird WM.Carcinogenic polycyclic aromatic hydrocarbonsIn: McQueen CA, Roberts R, (eds).Comprehensive Toxicology, Vol 14Elsevier: Oxford; 201085–124.
-
- Zhu H, Li Y, Trush MA. Characterization of benzo[a]pyrene quinone-induced toxicity to primary cultured bone marrow stromal cells from DBA/2 mice: potential role of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1995;130:108–120. - PubMed
-
- Bock KW, Köhle C. The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem. 2009;390:1225–1235. - PubMed
-
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

