Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury
- PMID: 22952165
- PMCID: PMC3437429
- DOI: 10.1136/bmjopen-2012-001606
Lung Injury Prevention with Aspirin (LIPS-A): a protocol for a multicentre randomised clinical trial in medical patients at high risk of acute lung injury
Abstract
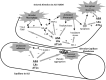
Introduction: Acute lung injury (ALI) is a devastating condition that places a heavy burden on public health resources. Although the need for effective ALI prevention strategies is increasingly recognised, no effective preventative strategies exist. The Lung Injury Prevention Study with Aspirin (LIPS-A) aims to test whether aspirin (ASA) could prevent and/or mitigate the development of ALI.
Methods and analysis: LIPS-A is a multicentre, double-blind, randomised clinical trial testing the hypothesis that the early administration of ASA will result in a reduced incidence of ALI in adult patients at high risk. This investigation will enrol 400 study participants from 14 hospitals across the USA. Conditional logistic regression will be used to test the primary hypothesis that early ASA administration will decrease the incidence of ALI.
Ethics and dissemination: Safety oversight will be under the direction of an independent Data and Safety Monitoring Board (DSMB). Approval of the protocol was obtained from the DSMB prior to enrolling the first study participant. Approval of both the protocol and informed consent documents were also obtained from the institutional review board of each participating institution prior to enrolling study participants at the respective site. In addition to providing important clinical and mechanistic information, this investigation will inform the scientific merit and feasibility of a phase III trial on ASA as an ALI prevention agent. The findings of this investigation, as well as associated ancillary studies, will be disseminated in the form of oral and abstract presentations at major national and international medical specialty meetings. The primary objective and other significant findings will also be presented in manuscript form. All final, published manuscripts resulting from this protocol will be submitted to Pub Med Central in accordance with the National Institute of Health Public Access Policy.
Figures

References
-
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–93 - PubMed
-
- Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–304 - PubMed
-
- Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:293–301 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
