Randomized controlled trial of meat compared with multimicronutrient-fortified cereal in infants and toddlers with high stunting rates in diverse settings
- PMID: 22952176
- PMCID: PMC3441111
- DOI: 10.3945/ajcn.112.041962
Randomized controlled trial of meat compared with multimicronutrient-fortified cereal in infants and toddlers with high stunting rates in diverse settings
Abstract
Background: Improved complementary feeding is cited as a critical factor for reducing stunting. Consumption of meats has been advocated, but its efficacy in low-resource settings has not been tested.
Objective: The objective was to test the hypothesis that daily intake of 30 to 45 g meat from 6 to 18 mo of age would result in greater linear growth velocity and improved micronutrient status in comparison with an equicaloric multimicronutrient-fortified cereal.
Design: This was a cluster randomized efficacy trial conducted in the Democratic Republic of Congo, Zambia, Guatemala, and Pakistan. Individual daily portions of study foods and education messages to enhance complementary feeding were delivered to participants. Blood tests were obtained at trial completion.
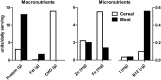
Results: A total of 532 (86.1%) and 530 (85.8%) participants from the meat and cereal arms, respectively, completed the study. Linear growth velocity did not differ between treatment groups: 1.00 (95% CI: 0.99, 1.02) and 1.02 (95% CI: 1.00, 1.04) cm/mo for the meat and cereal groups, respectively (P = 0.39). From baseline to 18 mo, stunting [length-for-age z score (LAZ) <-2.0] rates increased from ~33% to nearly 50%. Years of maternal education and maternal height were positively associated with linear growth velocity (P = 0.0006 and 0.003, respectively); LAZ at 6 mo was negatively associated (P < 0.0001). Anemia rates did not differ by group; iron deficiency was significantly lower in the cereal group.
Conclusion: The high rate of stunting at baseline and the lack of effect of either the meat or multiple micronutrient-fortified cereal intervention to reverse its progression argue for multifaceted interventions beginning in the pre- and early postnatal periods.
Trial registration: ClinicalTrials.gov NCT01084109.
Figures


References
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243–60 - PubMed
-
- Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125(3):e473–80 - PubMed
-
- Fenn B, Penny ME. Using the new World Health Organisation growth standards: differences from 3 countries. J Pediatr Gastroenterol Nutr 2008;46():316–21 - PubMed
-
- Umeta M, West CE, Verhoef H, Haidar J, Hautvast JG. Factors associated with stunting in infants aged 5-11 months in the Dodota-Sire District, rural Ethiopia. J Nutr 2003;133:1064–9 - PubMed
-
- World Health Organization Complementary feeding of young children in developing countries: A review of current scientific knowledge. Geneva, Switzerland: World Health Organization, 1998
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

