A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model
- PMID: 22952217
- PMCID: PMC3632398
- DOI: 10.1158/0008-5472.CAN-12-0895
A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model
Abstract
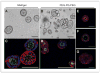
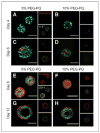
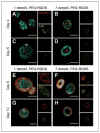
Better understanding of the biophysical and biochemical cues of the tumor extracellular matrix environment that influence metastasis may have important implications for new cancer therapeutics. Initial exploration into this question has used naturally derived protein matrices that suffer from variability, poor control over matrix biochemistry, and inability to modify the matrix biochemistry and mechanics. Here, we report the use of a synthetic polymer-based scaffold composed primarily of poly(ethylene glycol), or PEG, modified with bioactive peptides to study murine models of lung adenocarcinoma. In this study, we focus on matrix-derived influences on epithelial morphogenesis of a metastatic cell line (344SQ) that harbors mutations in Kras and p53 (trp53) and is prone to a microRNA-200 (miR-200)-dependent epithelial-mesenchymal transition (EMT) and metastasis. The modified PEG hydrogels feature biospecific cell adhesion and cell-mediated proteolytic degradation with independently adjustable matrix stiffness. 344SQ encapsulated in bioactive peptide-modified, matrix metalloproteinase-degradable PEG hydrogels formed lumenized epithelial spheres comparable to that seen with three-dimensional culture in Matrigel. Altering both matrix stiffness and the concentration of cell-adhesive ligand significantly influenced epithelial morphogenesis as manifest by differences in the extent of lumenization, in patterns of intrasphere apoptosis and proliferation, and in expression of epithelial polarity markers. Regardless of matrix composition, exposure to TGF-β induced a loss of epithelial morphologic features, shift in expression of EMT marker genes, and decrease in mir-200 levels consistent with EMT. Our findings help illuminate matrix-derived cues that influence epithelial morphogenesis and highlight the potential utility that this synthetic matrix-mimetic tool has for cancer biology.
©2012 AACR.
Conflict of interest statement
Figures




References
-
- Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

