Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3
- PMID: 22952242
- PMCID: PMC3476316
- DOI: 10.1074/jbc.M112.376111
Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3
Abstract
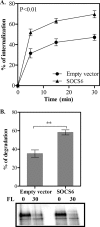
The receptor tyrosine kinase Flt3 is an important growth factor receptor in hematopoiesis, and gain-of-function mutations of the receptor contribute to the transformation of acute myeloid leukemia. SOCS6 (suppressor of cytokine signaling 6) is a member of the SOCS family of E3 ubiquitin ligases that can regulate receptor tyrosine kinase signal transduction. In this study, we analyzed the role of SOCS6 in Flt3 signal transduction. The results show that ligand stimulation of Flt3 can induce association of SOCS6 and Flt3 and tyrosine phosphorylation of SOCS6. Phosphopeptide fishing indicated that SOCS6 binds directly to phosphotyrosines 591 and 919 of Flt3. By using stably transfected Ba/F3 cells with Flt3 and/or SOCS6, we show that the presence of SOCS6 can enhance ubiquitination of Flt3, as well as internalization and degradation of the receptor. The presence of SOCS6 also induces weaker activation of Erk1/2, but not Akt, in transfected Ba/F3 and UT-7 cells and in OCI-AML-5 cells. The absence of SOCS6 promotes Ba/F3 and UT-7 cell proliferation induced by oncogenic internal tandem duplications of Flt3. Taken together, these results suggest that SOCS6 negatively regulates Flt3 activation, the downstream Erk signaling pathway, and cell proliferation.
Figures






Similar articles
-
FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia.Mol Oncol. 2013 Jun;7(3):402-18. doi: 10.1016/j.molonc.2012.11.003. Epub 2012 Nov 29. Mol Oncol. 2013. PMID: 23246379 Free PMC article.
-
Src-Like adaptor protein (SLAP) binds to the receptor tyrosine kinase Flt3 and modulates receptor stability and downstream signaling.PLoS One. 2012;7(12):e53509. doi: 10.1371/journal.pone.0053509. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300935 Free PMC article.
-
Suppressor of cytokine signaling 2 (SOCS2) associates with FLT3 and negatively regulates downstream signaling.Mol Oncol. 2013 Jun;7(3):693-703. doi: 10.1016/j.molonc.2013.02.020. Epub 2013 Mar 19. Mol Oncol. 2013. PMID: 23548639 Free PMC article.
-
The role of SRC family kinases in FLT3 signaling.Int J Biochem Cell Biol. 2019 Feb;107:32-37. doi: 10.1016/j.biocel.2018.12.007. Epub 2018 Dec 12. Int J Biochem Cell Biol. 2019. PMID: 30552988 Review.
-
Suppressor of cytokine signaling 6 in cancer development and therapy: Deciphering its emerging and suppressive roles.Cytokine Growth Factor Rev. 2022 Apr;64:21-32. doi: 10.1016/j.cytogfr.2022.02.001. Epub 2022 Feb 9. Cytokine Growth Factor Rev. 2022. PMID: 35210167 Review.
Cited by
-
SOCS6 promotes radiosensitivity and decreases cancer cell stemness in esophageal squamous cell carcinoma by regulating c-Kit ubiquitylation.Cancer Cell Int. 2021 Mar 12;21(1):165. doi: 10.1186/s12935-021-01859-2. Cancer Cell Int. 2021. PMID: 33712005 Free PMC article.
-
Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML.Blood. 2016 Oct 13;128(15):1944-1958. doi: 10.1182/blood-2016-04-708750. Epub 2016 Aug 18. Blood. 2016. PMID: 27540013 Free PMC article.
-
FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia.Mol Oncol. 2013 Jun;7(3):402-18. doi: 10.1016/j.molonc.2012.11.003. Epub 2012 Nov 29. Mol Oncol. 2013. PMID: 23246379 Free PMC article.
-
The Impact of FLT3 Mutations on the Development of Acute Myeloid Leukemias.Leuk Res Treatment. 2013;2013:275760. doi: 10.1155/2013/275760. Epub 2013 Jul 9. Leuk Res Treatment. 2013. PMID: 23936658 Free PMC article.
-
Activating FLT3 mutants show distinct gain-of-function phenotypes in vitro and a characteristic signaling pathway profile associated with prognosis in acute myeloid leukemia.PLoS One. 2014 Mar 7;9(3):e89560. doi: 10.1371/journal.pone.0089560. eCollection 2014. PLoS One. 2014. PMID: 24608088 Free PMC article.
References
-
- Masson K., Rönnstrand L. (2009) Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell. Signal. 21, 1717–1726 - PubMed
-
- Pawson T. (2004) Specificity in signal transduction: from phosphotyrosine-SH2 domain interactions to complex cellular systems. Cell 116, 191–203 - PubMed
-
- Levis M., Small D. (2003) FLT3: ITDoes matter in leukemia. Leukemia 17, 1738–1752 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

