Intrarenal localization of the plasma membrane ATP channel pannexin1
- PMID: 22952282
- PMCID: PMC3517631
- DOI: 10.1152/ajprenal.00206.2011
Intrarenal localization of the plasma membrane ATP channel pannexin1
Abstract
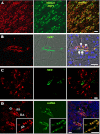
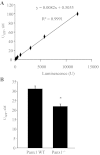
In the renal tubules, ATP released from epithelial cells stimulates purinergic receptors, regulating salt and water reabsorption. However, the mechanisms by which ATP is released into the tubular lumen are multifaceted. Pannexin1 (Panx1) is a newly identified. ubiquitously expressed protein that forms connexin-like channels in the plasma membrane, which have been demonstrated to function as a mechanosensitive ATP conduit. Here, we report on the localization of Panx1 in the mouse kidney. Using immunofluorescence, strong Panx1 expression was observed in renal tubules, including proximal tubules, thin descending limbs, and collecting ducts, along their apical cell membranes. In the renal vasculature, Panx1 expression was localized to vascular smooth muscle cells in renal arteries, including the afferent and efferent arterioles. Additionally, we tested whether Panx1 channels expressed in renal epithelial cells facilitate luminal ATP release by measuring the ATP content of urine samples freshly collected from wild-type and Panx1(-/-) mice. Urinary ATP levels were reduced by 30% in Panx1(-/-) compared with wild-type mice. These results suggest that Panx1 channels in the kidney may regulate ATP release and via purinergic signaling may participate in the control of renal epithelial fluid and electrolyte transport and vascular functions.
Figures


References
-
- Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287: F789–F796, 2004 - PubMed
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004 - PubMed
-
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83: 706–716, 2004 - PubMed
-
- Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology 21: 103–114, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

