Ex vivo stimulation of whole blood as a means to determine glucocorticoid sensitivity
- PMID: 22952414
- PMCID: PMC3430009
- DOI: 10.2147/JIR.S33569
Ex vivo stimulation of whole blood as a means to determine glucocorticoid sensitivity
Abstract
Purpose: Glucocorticoids are commonly prescribed to treat a number of diseases including the majority of inflammatory diseases. Despite considerable interpersonal variability in response to glucocorticoids, an insensitivity rate of about 30%, and the risk of adverse side effects of glucocorticoid therapy, currently no assay is performed to determine sensitivity.
Patients and methods: Here we propose a whole blood ex vivo stimulation assay to interrogate known glucocorticoid receptor (GR) up- and downregulated genes to indicate glucocorticoid sensitivity. We have chosen to employ real-time PCR in order to provide a relatively fast and inexpensive assay.
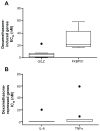
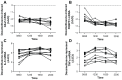
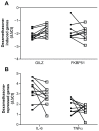
Results: We show that the GR-regulated genes, GILZ and FKBP51, are upregulated in whole blood by treatment with dexamethasone and that LPS-induction of cytokines (IL-6 and TNFα) are repressed by dexamethasone in a dose responsive manner. There is considerable interpersonal variability in the maximum induction of these genes but little variation in the EC(50) and IC(50) concentrations. The regulation of the GR-induced genes differs throughout the day whereas the suppression of LPS-induced cytokines is not as sensitive to time of day.
Conclusion: In all, this assay would provide a method to determine glucocorticoid receptor responsiveness in whole blood.
Keywords: FKBP51; GILZ; cytokines; gene regulation; glucocorticoid responsiveness; nuclear receptor.
Figures


References
-
- van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105–111. - PubMed
-
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. - PubMed
-
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81(3):1269–1304. - PubMed
-
- Schoneveld OJ, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680(2):114–128. - PubMed
-
- Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1–3):51–62. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

