Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients
- PMID: 22952439
- PMCID: PMC3429397
- DOI: 10.1371/journal.pmed.1001300
Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients
Abstract
Background: Treatment of multidrug resistant tuberculosis (MDR-TB) is lengthy, toxic, expensive, and has generally poor outcomes. We undertook an individual patient data meta-analysis to assess the impact on outcomes of the type, number, and duration of drugs used to treat MDR-TB.
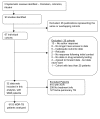
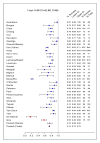
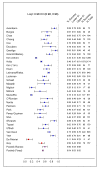
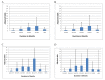
Methods and findings: Three recent systematic reviews were used to identify studies reporting treatment outcomes of microbiologically confirmed MDR-TB. Study authors were contacted to solicit individual patient data including clinical characteristics, treatment given, and outcomes. Random effects multivariable logistic meta-regression was used to estimate adjusted odds of treatment success. Adequate treatment and outcome data were provided for 9,153 patients with MDR-TB from 32 observational studies. Treatment success, compared to failure/relapse, was associated with use of: later generation quinolones, (adjusted odds ratio [aOR]: 2.5 [95% CI 1.1-6.0]), ofloxacin (aOR: 2.5 [1.6-3.9]), ethionamide or prothionamide (aOR: 1.7 [1.3-2.3]), use of four or more likely effective drugs in the initial intensive phase (aOR: 2.3 [1.3-3.9]), and three or more likely effective drugs in the continuation phase (aOR: 2.7 [1.7-4.1]). Similar results were seen for the association of treatment success compared to failure/relapse or death: later generation quinolones, (aOR: 2.7 [1.7-4.3]), ofloxacin (aOR: 2.3 [1.3-3.8]), ethionamide or prothionamide (aOR: 1.7 [1.4-2.1]), use of four or more likely effective drugs in the initial intensive phase (aOR: 2.7 [1.9-3.9]), and three or more likely effective drugs in the continuation phase (aOR: 4.5 [3.4-6.0]).
Conclusions: In this individual patient data meta-analysis of observational data, improved MDR-TB treatment success and survival were associated with use of certain fluoroquinolones, ethionamide, or prothionamide, and greater total number of effective drugs. However, randomized trials are urgently needed to optimize MDR-TB treatment. Please see later in the article for the Editors' Summary.
Conflict of interest statement
JR is a Consultant for bioMérieux. WWY has been indirectly sponsored to participate in International Conferences by GlaxoSmithKline and Pfizer in the last 3 years. CDM is on the Scientific Advisory Board for Otsuka pharmaceuticals development of OPC67683 (Delaminid), a new anti-TB compound. SK received salary support from the Eli Lilly Foundation as part of funding for the activities of Partners In Health by the Foundation's MDR-TB Partnership. This funder was not involved in the study design; collection, analysis and interpretation of data; writing of the paper; and/or decision to submit for publication. The Partners In Health project in Tomsk received funding from Mr. Tom White, the Open Society Institute, the Bill and Melinda Gates Foundation, and the Global Fund to fight AIDS, Tuberculosis and Malaria. None of these funders were involved in the study design; collection, analysis and interpretation of data; writing of the paper; and/or decision to submit for publication. KD is an unpaid, volunteer member of the New Diagnostics Working Group (NDWG), formed of members of the Stop TB Partnership. The Secretariat of the NDWG is hosted by FIND (Foundation for New Innovative Diagnostics). JB was working as consultant for Otsuka Pharmaceutical for the implementation of clinical trial in Peru. JB was co PI of a NIH grant in Peru, Epidemiology of Tuberculosis. MP and GP are members of the Editorial Board of
Figures
References
-
- World Health Organization (2008) Guidelines for the programmatic management of drug resistant tuberculosis - emergency update. World Health Organization 247 p.
-
- Caminero JA, Sotgiu G, Zumla A, Migliori GB (2010) Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet 10: 621–629. - PubMed
-
- Akcakir Y (2010) Correlates of treatment outcomes of multidrug-resistant tuberculosis (MDR-TB): a systematic review and meta-analysis [PhD dissertation]. Montreal: Department of Epidemiology & Biostatistics, McGill University. 110 p.
-
- Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, et al. (2009) Treatment outcomes among patients with multidrug-resistant tuberculosis:systematic review and meta-analysis. Lancet Infect Dis 9: 153–161. - PubMed
-
- Johnston JC, Shahidi NC, Sadatsafavi M, FitzGerald JM (2009) Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One 4: e6914 doi:10.1371/journal.pone.0006914. - DOI - PMC - PubMed

