Measles immune suppression: lessons from the macaque model
- PMID: 22952446
- PMCID: PMC3431343
- DOI: 10.1371/journal.ppat.1002885
Measles immune suppression: lessons from the macaque model
Abstract
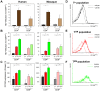
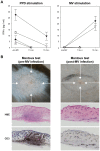
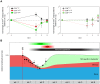
Measles remains a significant childhood disease, and is associated with a transient immune suppression. Paradoxically, measles virus (MV) infection also induces robust MV-specific immune responses. Current hypotheses for the mechanism underlying measles immune suppression focus on functional impairment of lymphocytes or antigen-presenting cells, caused by infection with or exposure to MV. We have generated stable recombinant MVs that express enhanced green fluorescent protein, and remain virulent in non-human primates. By performing a comprehensive study of virological, immunological, hematological and histopathological observations made in animals euthanized at different time points after MV infection, we developed a model explaining measles immune suppression which fits with the "measles paradox". Here we show that MV preferentially infects CD45RA(-) memory T-lymphocytes and follicular B-lymphocytes, resulting in high infection levels in these populations. After the peak of viremia MV-infected lymphocytes were cleared within days, followed by immune activation and lymph node enlargement. During this period tuberculin-specific T-lymphocyte responses disappeared, whilst strong MV-specific T-lymphocyte responses emerged. Histopathological analysis of lymphoid tissues showed lymphocyte depletion in the B- and T-cell areas in the absence of apoptotic cells, paralleled by infiltration of T-lymphocytes into B-cell follicles and reappearance of proliferating cells. Our findings indicate an immune-mediated clearance of MV-infected CD45RA(-) memory T-lymphocytes and follicular B-lymphocytes, which causes temporary immunological amnesia. The rapid oligoclonal expansion of MV-specific lymphocytes and bystander cells masks this depletion, explaining the short duration of measles lymphopenia yet long duration of immune suppression.
Conflict of interest statement
A.D.M.E. Osterhaus wishes to declare, for the avoidance of any misunderstanding on competing interests, that he co-founded and is chief scientific officer of Viroclinics Biosciences, a company set up in collaboration with Erasmus MC. However, for clarification, no materials or support were received from the company, and no agreements were in place concerning the execution or publication of this work. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials. The authors have no additional financial interests.
Figures


References
-
- Beckford AP, Kaschula RO, Stephen C (1985) Factors associated with fatal cases of measles. A retrospective autopsy study. S Afr Med J 68: 858–863. - PubMed
-
- Akramuzzaman SM, Cutts FT, Wheeler JG, Hossain MJ (2000) Increased childhood morbidity after measles is short-term in urban Bangladesh. Am J Epidemiol 151: 723–735. - PubMed
-
- Shanks GD, Lee SE, Howard A, Brundage JF (2011) Extreme mortality after first introduction of measles virus to the polynesian island of Rotuma, 1911. Am J Epidemiol 173: 1211–1222. - PubMed
-
- Von Pirquet CE (1908) Das Verhalten der kutanen Tuberkulin-reaktion während der Masern. Dtsch Med Wochenschr 34: 1297–1300.
-
- Tamashiro VG, Perez HH, Griffin DE (1987) Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J 6: 451–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

