Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization
- PMID: 22952616
- PMCID: PMC3428339
- DOI: 10.1371/journal.pone.0042842
Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization
Abstract
The recently identified type VI secretion system (T6SS) of proteobacteria has been shown to promote pathogenicity, competitive advantage over competing microorganisms, and adaptation to environmental perturbation. By detailed phenotypic characterization of loss-of-function mutants, in silico, in vitro and in vivo analyses, we provide evidence that the enteric pathogen, Campylobacter jejuni, possesses a functional T6SS and that the secretion system exerts pleiotropic effects on two crucial processes--survival in a bile salt, deoxycholic acid (DCA), and host cell adherence and invasion. The expression of T6SS during initial exposure to the upper range of physiological levels of DCA (0.075%-0.2%) was detrimental to C. jejuni proliferation, whereas down-regulation or inactivation of T6SS enabled C. jejuni to resist this effect. The C. jejuni multidrug efflux transporter gene, cmeA, was significantly up-regulated during the initial exposure to DCA in the wild type C. jejuni relative to the T6SS-deficient strains, suggesting that inhibition of proliferation is the consequence of T6SS-mediated DCA influx. A sequential modulation of the efflux transporter activity and the T6SS represents, in part, an adaptive mechanism for C. jejuni to overcome this inhibitory effect, thereby ensuring its survival. C. jejuni T6SS plays important roles in host cell adhesion and invasion as T6SS inactivation resulted in a reduction of adherence to and invasion of in vitro cell lines, while over-expression of a hemolysin co-regulated protein, which encodes a secreted T6SS component, greatly enhanced these processes. When inoculated into B6.129P2-IL-10(tm1Cgn) mice, the T6SS-deficient C. jejuni strains did not effectively establish persistent colonization, indicating that T6SS contributes to colonization in vivo. Taken together, our data demonstrate the importance of bacterial T6SS in host cell adhesion, invasion, colonization and, for the first time to our knowledge, adaptation to DCA, providing new insights into the role of T6SS in C. jejuni pathogenesis.
Conflict of interest statement
Figures




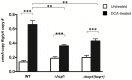



References
-
- Records AR (2011) The type VI secretion system: a multipurpose delivery system with a phage-like machinery. Mol Plant Microbe Interact 24: 751–757. - PubMed
-
- Zheng J, Leung KY (2007) Dissection of a type VI secretion system in Edwardsiella tarda . Mol Microbiol 66: 1192–1206. - PubMed
-
- Sarris PF, Zoumadakis C, Panopoulos NJ, Scoulica EV (2010) Distribution of the putative type VI secretion system core genes in Klebsiella spp. Infect Genet Evol 11: 157–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

