Characterization of omental immune aggregates during establishment of a latent gammaherpesvirus infection
- PMID: 22952645
- PMCID: PMC3430671
- DOI: 10.1371/journal.pone.0043196
Characterization of omental immune aggregates during establishment of a latent gammaherpesvirus infection
Abstract
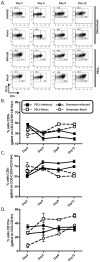
Herpesviruses are characterized by their ability to establish lifelong latent infection. The gammaherpesvirus subfamily is distinguished by lymphotropism, establishing and maintaining latent infection predominantly in B lymphocytes. Consequently, gammaherpesvirus pathogenesis is closely linked to normal B cell physiology. Murine gammaherpesvirus 68 (MHV68) pathogenesis in laboratory mice has been extensively studied as a model system to gain insights into the nature of gammaherpesvirus infection in B cells and their associated lymphoid compartments. In addition to B cells, MHV68 infection of macrophages contributes significantly to the frequency of viral genome-positive cells in the peritoneal cavity throughout latency. The omentum, a sheet of richly-vascularized adipose tissue, resides in the peritoneal cavity and contains clusters of immune cell aggregates termed milky spots. Although the value of the omentum in surgical wound-healing has long been appreciated, the unique properties of this tissue and its contribution to both innate and adaptive immunity have only recently been recognized. To determine whether the omentum plays a role in gammaherpesvirus pathogenesis we examined this site during early MHV68 infection and long-term latency. Following intraperitoneal infection, immune aggregates within the omentum expanded in size and number and contained virus-infected cells. Notably, a germinal-center B cell population appeared in the omentum of infected animals with earlier kinetics and greater magnitude than that observed in the spleen. Furthermore, the omentum harbored a stable frequency of viral genome-positive cells through early and into long-term latency, while removal of the omentum prior to infection resulted in a slight decrease in the establishment of splenic latency following intraperitoneal infection. These data provide the first evidence that the omentum is a site of chronic MHV68 infection that may contribute to the maintenance of chronic infection.
Conflict of interest statement
Figures







References
-
- Barton E, Mandal P, Speck SH (2011) Pathogenesis and host control of gammaherpesviruses: lessons from the mouse. Annu Rev Immunol 29: 351–397. - PubMed
-
- Peacock JW, Bost KL (2000) Infection of intestinal epithelial cells and development of systemic disease following gastric instillation of murine gammaherpesvirus-68. J Gen Virol 81: 421–429. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

