Differential effects of two fermentable carbohydrates on central appetite regulation and body composition
- PMID: 22952656
- PMCID: PMC3430697
- DOI: 10.1371/journal.pone.0043263
Differential effects of two fermentable carbohydrates on central appetite regulation and body composition
Abstract
Background: Obesity is rising at an alarming rate globally. Different fermentable carbohydrates have been shown to reduce obesity. The aim of the present study was to investigate if two different fermentable carbohydrates (inulin and β-glucan) exert similar effects on body composition and central appetite regulation in high fat fed mice.
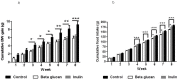
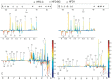
Methodology/principal findings: Thirty six C57BL/6 male mice were randomized and maintained for 8 weeks on a high fat diet containing 0% (w/w) fermentable carbohydrate, 10% (w/w) inulin or 10% (w/w) β-glucan individually. Fecal and cecal microbial changes were measured using fluorescent in situ hybridization, fecal metabolic profiling was obtained by proton nuclear magnetic resonance ((1)H NMR), colonic short chain fatty acids were measured by gas chromatography, body composition and hypothalamic neuronal activation were measured using magnetic resonance imaging (MRI) and manganese enhanced MRI (MEMRI), respectively, PYY (peptide YY) concentration was determined by radioimmunoassay, adipocyte cell size and number were also measured. Both inulin and β-glucan fed groups revealed significantly lower cumulative body weight gain compared with high fat controls. Energy intake was significantly lower in β-glucan than inulin fed mice, with the latter having the greatest effect on total adipose tissue content. Both groups also showed an increase in the numbers of Bifidobacterium and Lactobacillus-Enterococcus in cecal contents as well as feces. β-Glucan appeared to have marked effects on suppressing MEMRI associated neuronal signals in the arcuate nucleus, ventromedial hypothalamus, paraventricular nucleus, periventricular nucleus and the nucleus of the tractus solitarius, suggesting a satiated state.
Conclusions/significance: Although both fermentable carbohydrates are protective against increased body weight gain, the lower body fat content induced by inulin may be metabolically advantageous. β-Glucan appears to suppress neuronal activity in the hypothalamic appetite centers. Differential effects of fermentable carbohydrates open new possibilities for nutritionally targeting appetite regulation and body composition.
Conflict of interest statement
Figures


References
-
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. - PubMed
-
- Kleessen B, Hartmann L, Blaut M (2001) Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br J Nutr 86: 291–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

