LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing
- PMID: 22952743
- PMCID: PMC3429492
- DOI: 10.1371/journal.pone.0043709
LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing
Abstract
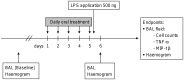
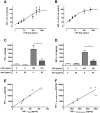
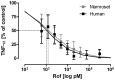
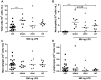
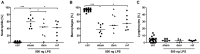
Increasing incidence and substantial morbidity and mortality of respiratory diseases requires the development of new human-specific anti-inflammatory and disease-modifying therapeutics. Therefore, new predictive animal models that closely reflect human lung pathology are needed. In the current study, a tiered acute lipopolysaccharide (LPS)-induced inflammation model was established in marmoset monkeys (Callithrix jacchus) to reflect crucial features of inflammatory lung diseases. Firstly, in an ex vivo approach marmoset and, for the purposes of comparison, human precision-cut lung slices (PCLS) were stimulated with LPS in the presence or absence of the phosphodiesterase-4 (PDE4) inhibitor roflumilast. Pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and macrophage inflammatory protein-1 beta (MIP-1β) were measured. The corticosteroid dexamethasone was used as treatment control. Secondly, in an in vivo approach marmosets were pre-treated with roflumilast or dexamethasone and unilaterally challenged with LPS. Ipsilateral bronchoalveolar lavage (BAL) was conducted 18 hours after LPS challenge. BAL fluid was processed and analyzed for neutrophils, TNF-α, and MIP-1β. TNF-α release in marmoset PCLS correlated significantly with human PCLS. Roflumilast treatment significantly reduced TNF-α secretion ex vivo in both species, with comparable half maximal inhibitory concentration (IC(50)). LPS instillation into marmoset lungs caused a profound inflammation as shown by neutrophilic influx and increased TNF-α and MIP-1β levels in BAL fluid. This inflammatory response was significantly suppressed by roflumilast and dexamethasone. The close similarity of marmoset and human lungs regarding LPS-induced inflammation and the significant anti-inflammatory effect of approved pharmaceuticals assess the suitability of marmoset monkeys to serve as a promising model for studying anti-inflammatory drugs.
Conflict of interest statement
Figures

Similar articles
-
Anti-neutrophilic inflammatory activity of ASP3258, a novel phosphodiesterase type 4 inhibitor.Int Immunopharmacol. 2012 Jan;12(1):59-63. doi: 10.1016/j.intimp.2011.10.011. Epub 2011 Oct 29. Int Immunopharmacol. 2012. PMID: 22041526
-
Effects of tadalafil (PDE5 inhibitor) and roflumilast (PDE4 inhibitor) on airway reactivity and markers of inflammation in ovalbumin-induced airway hyperresponsiveness in guinea pigs.J Physiol Pharmacol. 2017 Oct;68(5):721-730. J Physiol Pharmacol. 2017. PMID: 29375047
-
Roflumilast reduces the number of lung adenocarcinomas, inflammation, and emphysema in a smoking-induced mouse model.BMC Pulm Med. 2025 May 26;25(1):262. doi: 10.1186/s12890-025-03730-w. BMC Pulm Med. 2025. PMID: 40420271 Free PMC article.
-
The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease.Pulm Pharmacol Ther. 2010 Aug;23(4):235-56. doi: 10.1016/j.pupt.2010.03.011. Epub 2010 Apr 7. Pulm Pharmacol Ther. 2010. PMID: 20381629 Review.
-
Scope of adjuvant therapy using roflumilast, a PDE-4 inhibitor against COVID-19.Pulm Pharmacol Ther. 2021 Feb;66:101978. doi: 10.1016/j.pupt.2020.101978. Epub 2020 Nov 28. Pulm Pharmacol Ther. 2021. PMID: 33259924 Free PMC article. Review.
Cited by
-
Low-dose endotoxin inhalation in healthy volunteers--a challenge model for early clinical drug development.BMC Pulm Med. 2013 Mar 28;13:19. doi: 10.1186/1471-2466-13-19. BMC Pulm Med. 2013. PMID: 23537365 Free PMC article. Clinical Trial.
-
Airway hyper-responsiveness in lipopolysaccharide-challenged common marmosets (Callithrix jacchus).Clin Sci (Lond). 2014 Jan;126(2):155-62. doi: 10.1042/CS20130101. Clin Sci (Lond). 2014. PMID: 23879175 Free PMC article.
-
Use of precision cut lung slices as a translational model for the study of lung biology.Respir Res. 2019 Jul 19;20(1):162. doi: 10.1186/s12931-019-1131-x. Respir Res. 2019. PMID: 31324219 Free PMC article. Review.
-
Oxypeucedanin relieves LPS-induced acute lung injury by inhibiting the inflammation and maintaining the integrity of the lung air-blood barrier.Aging (Albany NY). 2022 Aug 18;14(16):6626-6641. doi: 10.18632/aging.204235. Epub 2022 Aug 18. Aging (Albany NY). 2022. PMID: 35985771 Free PMC article.
-
Marmosets: Welfare, Ethical Use, and IACUC/Regulatory Considerations.ILAR J. 2020 Dec 31;61(2-3):167-178. doi: 10.1093/ilar/ilab003. ILAR J. 2020. PMID: 33620069 Free PMC article. Review.
References
-
- de Marco R, Accordini S, Cerveri I, Corsico A, Anto JM, et al. (2007) Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med 175(1): 32–9. - PubMed
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353(16): 1685–93. - PubMed
-
- Barnes PJ (2004) Alveolar macrophages as orchestrators of COPD. COPD 1(1): 59–70. - PubMed
-
- Bundschuh DS, Eltze M, Barsig J, Wollin L, Hatzelmann A, et al. (2001) In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 297(1): 280–90. - PubMed
-
- Hicks A, Kourteva G, Hilton H, Li H, Lin TA, et al. (2010) Cellular and molecular characterization of ozone-induced pulmonary inflammation in the Cynomolgus monkey. Inflammation 33(3): 144–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

