Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection
- PMID: 22952756
- PMCID: PMC3432055
- DOI: 10.1371/journal.pone.0043754
Plasma HIV viral rebound following protocol-indicated cessation of ART commenced in primary and chronic HIV infection
Abstract
Objectives: The magnitude of HIV viral rebound following ART cessation has consequences for clinical outcome and onward transmission. We compared plasma viral load (pVL) rebound after stopping ART initiated in primary (PHI) and chronic HIV infection (CHI).
Design: Two populations with protocol-indicated ART cessation from SPARTAC (PHI, n = 182) and SMART (CHI, n = 1450) trials.
Methods: Time for pVL to reach pre-ART levels after stopping ART was assessed in PHI using survival analysis. Differences in pVL between PHI and CHI populations 4 weeks after stopping ART were examined using linear and logistic regression. Differences in pVL slopes up to 48 weeks were examined using linear mixed models and viral burden was estimated through a time-averaged area-under-pVL curve. CHI participants were categorised by nadir CD4 at ART stop.
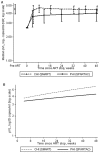
Results: Of 171 PHI participants, 71 (41.5%) rebounded to pre-ART pVL levels, at a median of 50 (95% CI 48-51) weeks after stopping ART. Four weeks after stopping treatment, although the proportion with pVL ≥ 400 copies/ml was similar (78% PHI versus 79% CHI), levels were 0.45 (95% CI 0.26-0.64) log(10) copies/ml lower for PHI versus CHI, and remained lower up to 48 weeks. Lower CD4 nadir in CHI was associated with higher pVL after ART stop. Rebound for CHI participants with CD4 nadir >500 cells/mm(3) was comparable to that experienced by PHI participants.
Conclusions: Stopping ART initiated in PHI and CHI was associated with viral rebound to levels conferring increased transmission risk, although the level of rebound was significantly lower and sustained in PHI compared to CHI.
Conflict of interest statement
Figures

References
-
- Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, et al. (2006) CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 368: 459–65. - PubMed
-
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. (2006) CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine 355: 2283–96. - PubMed
-
- Oxenius A, Price DA, Gunthard HF, Dawson SJ, Fagard C, et al. (2002) Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proceedings of the National Academy of Sciences of the United States of America 99: 13747–52. - PMC - PubMed
-
- Wit FW, Blanckenberg DH, Brinkman K, Prins JM, van der Ende ME, et al. (2000) Safety of long-term interruption of successful antiretroviral therapy: the ATHENA cohort study. AIDS 19: 345–8. - PubMed
-
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, et al. (2000) Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. New England Journal of Medicine 342: 921–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 069598/Z/02/B/WT_/Wellcome Trust/United Kingdom
- U01-AI042170/AI/NIAID NIH HHS/United States
- U01 AI068641/AI/NIAID NIH HHS/United States
- WT069598MA/WT_/Wellcome Trust/United Kingdom
- MC_U122886352/MRC_/Medical Research Council/United Kingdom
- G108/626/MRC_/Medical Research Council/United Kingdom
- U01-AI046362/AI/NIAID NIH HHS/United States
- R01 AI046995/AI/NIAID NIH HHS/United States
- U01-AI068641/AI/NIAID NIH HHS/United States
- U01 AI046362/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- U01 AI042170/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

