Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice
- PMID: 22952870
- PMCID: PMC3428308
- DOI: 10.1371/journal.pone.0044053
Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice
Abstract
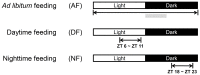
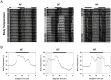
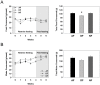
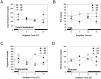
In modern society, growing numbers of people are engaged in various forms of shift works or trans-meridian travels. Such circadian misalignment is known to disturb endogenous diurnal rhythms, which may lead to harmful physiological consequences including metabolic syndrome, obesity, cancer, cardiovascular disorders, and gastric disorders as well as other physical and mental disorders. However, the precise mechanism(s) underlying these changes are yet unclear. The present work, therefore examined the effects of 6 h advance or delay of usual meal time on diurnal rhythmicities in home cage activity (HCA), body temperature (BT), blood metabolic markers, glucose homeostasis, and expression of genes that are involved in cholesterol homeostasis by feeding young adult male mice in a time-restrictive manner. Delay of meal time caused locomotive hyperactivity in a significant portion (42%) of subjects, while 6 h advance caused a torpor-like symptom during the late scotophase. Accordingly, daily rhythms of blood glucose and triglyceride were differentially affected by time-restrictive feeding regimen with concurrent metabolic alterations. Along with these physiological changes, time-restrictive feeding also influenced the circadian expression patterns of low density lipoprotein receptor (LDLR) as well as most LDLR regulatory factors. Strikingly, chronic advance of meal time induced insulin resistance, while chronic delay significantly elevated blood glucose levels. Taken together, our findings indicate that persistent shifts in usual meal time impact the diurnal rhythms of carbohydrate and lipid metabolisms in addition to HCA and BT, thereby posing critical implications for the health and diseases of shift workers.
Conflict of interest statement
Figures



References
-
- Dunlap JC, Loros JJ, DeCoursey PJ (2004) Chronobiology: Biological Timekeeping. Sinanuer Associates Inc, Sunderland, 406p.
-
- Refinetti R (2006) Circadian Physiology, 2nd ed. Taylor & Francis, Boca Raton, 667p.
-
- Szosland D (2010) Shift work and metabolic syndrome, diabetes mellitus and ischaemic heart disease. Int J Occup Med Environ Health 23: 287–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

