Telomere dynamics and homeostasis in a transmissible cancer
- PMID: 22952882
- PMCID: PMC3430654
- DOI: 10.1371/journal.pone.0044085
Telomere dynamics and homeostasis in a transmissible cancer
Abstract
Background: Devil Facial Tumour Disease (DFTD) is a unique clonal cancer that threatens the world's largest carnivorous marsupial, the Tasmanian devil (Sarcophilus harrisii) with extinction. This transmissible cancer is passed between individual devils by cell implantation during social interactions. The tumour arose in a Schwann cell of a single devil over 15 years ago and since then has expanded clonally, without showing signs of replicative senescence; in stark contrast to a somatic cell that displays a finite capacity for replication, known as the "Hayflick limit".
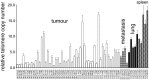
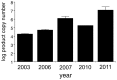
Methodology/principal findings: In the present study we investigate the role of telomere length, measured as Telomere Copy Number (TCN), and telomerase and shelterin gene expression, as well as telomerase activity in maintaining hyperproliferation of Devil Facial Tumour (DFT) cells. Our results show that DFT cells have short telomeres. DFTD TCN does not differ between geographic regions or between strains. However, TCN has increased over time. Unlimited cell proliferation is likely to have been achieved through the observed up-regulation of the catalytic subunit of telomerase (TERT) and concomitant activation of telomerase. Up-regulation of the central component of shelterin, the TRF1-intercating nuclear factor 2 (TINF2) provides DFT a mechanism for telomere length homeostasis. The higher expression of both TERT and TINF2 may also protect DFT cells from genomic instability and enhance tumour proliferation.
Conclusions/significance: DFT cells appear to monitor and regulate the length of individual telomeres: i.e. shorter telomeres are elongated by up-regulation of telomerase-related genes; longer telomeres are protected from further elongation by members of the shelterin complex, which may explain the lack of spatial and strain variation in DFT telomere copy number. The observed longitudinal increase in gene expression in DFT tissue samples and telomerase activity in DFT cell lines might indicate a selection for more stable tumours with higher proliferative potential.
Conflict of interest statement
Figures




References
-
- McCallum H (2008) Tasmanian devil facial tumour disease: lessons for conservation biology. Trends in Ecology and Evolution 23: 631–637. - PubMed
-
- Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, et al. (2006) Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation 131: 307–324.
-
- McCallum H, Tompkins DM, Jones M, Lachish S, Marvanek S, et al. (2007) Distribution and impacts of Tasmanian devil facial tumor disease. EcoHealth 4: 318–325.
-
- IUCN IUfCoN (2011) Red list of threatened species. Version 3.1. . IUCN,Gland, Switzerland Available from wwwiucnredlistorg (accessed January 2011).
-
- Hamede R, Lachish S, Belov K, Woods G, Kreiss A, et al. (2012) Reduced Effect of Tasmanian Devil Facial Tumor Disease at the Disease Front. Conservation Biology 26: 124–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

