Development of a novel molecular sensor for imaging estrogen receptor-coactivator protein-protein interactions
- PMID: 22952913
- PMCID: PMC3429467
- DOI: 10.1371/journal.pone.0044160
Development of a novel molecular sensor for imaging estrogen receptor-coactivator protein-protein interactions
Abstract
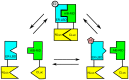
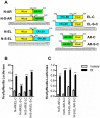
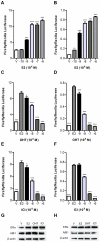
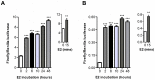
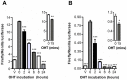
Anti-estrogens, in particular tissue selective anti-estrogens, have been the bedrock of adjuvant therapy for patients with estrogen receptor alpha (ERα) positive breast cancer. Though current therapies have greatly enhanced patient prognosis, there continues to be an impetus for the development of improved anti-estrogens. ERα is a nuclear receptor transcription factor which activates gene expression through the recruitment of transcriptional coactivator proteins. The SRC family of coactivators, which includes AIB1, has been shown to be of particular importance for ERα mediated transcription. ERα-AIB1 interactions are indicative of gene expression and are inhibited by anti-estrogen treatment. We have exploited the interaction between ERα and AIB1 as a novel method for imaging ERα activity using a split luciferase molecular sensor. By producing a range of ERα ligand binding domain (ER-LBD) and AIB1 nuclear receptor interacting domain (AIB-RID) N- and C-terminal firefly luciferase fragment fusion proteins, constructs which exhibited more than a 10-fold increase in luciferase activity with E2 stimulation were identified. The specificity of the E2-stimulated luciferase activity to ERα-AIB1 interaction was validated through Y537S and L539/540A ER-LBD fusion protein mutants. The primed nature of the split luciferase assay allowed changes in ERα activity, with respect to the protein-protein interactions preceding transcription, to be assessed soon after drug treatment. The novel assay split luciferase detailed in this report enabled modulation of ERα activity to be sensitively imaged in vitro and in living subjects and potentially holds much promise for imaging the efficacy of novel ERα specific therapies.
Conflict of interest statement
Figures

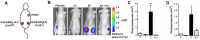
Similar articles
-
Breast cancer-derived M543V mutation in helix 12 of estrogen receptor alpha inverts response to estrogen and SERMs.Breast Cancer Res Treat. 2010 Apr;120(3):761-8. doi: 10.1007/s10549-009-0437-7. Epub 2009 Jun 13. Breast Cancer Res Treat. 2010. PMID: 19526339
-
Estrogen receptor α L543A,L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity.J Biol Chem. 2013 Jul 19;288(29):21105-21116. doi: 10.1074/jbc.M113.463455. Epub 2013 Jun 3. J Biol Chem. 2013. PMID: 23733188 Free PMC article.
-
In vitro and in vivo molecular imaging of estrogen receptor α and β homo- and heterodimerization: exploration of new modes of receptor regulation.Mol Endocrinol. 2011 Dec;25(12):2029-40. doi: 10.1210/me.2011-1145. Epub 2011 Nov 3. Mol Endocrinol. 2011. PMID: 22052998 Free PMC article.
-
Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review.
-
The role of SRC-3 in human breast cancer.Nat Rev Clin Oncol. 2010 Feb;7(2):83-9. doi: 10.1038/nrclinonc.2009.219. Epub 2009 Dec 22. Nat Rev Clin Oncol. 2010. PMID: 20027190 Review.
Cited by
-
The nuclear receptor 4A family members: mediators in human disease and autophagy.Cell Mol Biol Lett. 2020 Nov 3;25(1):48. doi: 10.1186/s11658-020-00241-w. Cell Mol Biol Lett. 2020. PMID: 33292165 Free PMC article. Review.
-
Proteomic profiling identifies key coactivators utilized by mutant ERα proteins as potential new therapeutic targets.Oncogene. 2018 Aug;37(33):4581-4598. doi: 10.1038/s41388-018-0284-2. Epub 2018 May 11. Oncogene. 2018. PMID: 29748621 Free PMC article.
-
Luciferase fragment complementation imaging in preclinical cancer studies.Oncoscience. 2014 Jun 1;1(5):310-25. doi: 10.18632/oncoscience.45. eCollection 2014. Oncoscience. 2014. PMID: 25594026 Free PMC article. Review.
-
Efficient Recombinase-Mediated Cassette Exchange in hPSCs to Study the Hepatocyte Lineage Reveals AAVS1 Locus-Mediated Transgene Inhibition.Stem Cell Reports. 2015 Nov 10;5(5):918-931. doi: 10.1016/j.stemcr.2015.09.004. Epub 2015 Oct 8. Stem Cell Reports. 2015. PMID: 26455413 Free PMC article.
References
-
- Ferlay J SH, Bray F, Forman D, Mathers C, Parkin DM (2010) GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010.
-
- Ali S, Buluwela L, Coombes RC (2011) Antiestrogens and Their Therapeutic Applications in Breast Cancer and Other Diseases. Annual Review of Medicine 62: 217–232. - PubMed
-
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ (2001) Nuclear Receptors and Lipid Physiology: Opening the X-Files. Science 294: 1866–1870. - PubMed
-
- Hartman J, Ström A, Gustafsson J-Å (2009) Estrogen receptor beta in breast cancer–Diagnostic and therapeutic implications. Steroids 74: 635–641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

