Endoplasmic reticulum sorting and kinesin-1 command the targeting of axonal GABAB receptors
- PMID: 22952914
- PMCID: PMC3428321
- DOI: 10.1371/journal.pone.0044168
Endoplasmic reticulum sorting and kinesin-1 command the targeting of axonal GABAB receptors
Abstract
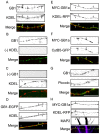
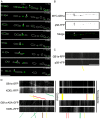
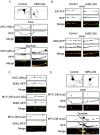
In neuronal cells the intracellular trafficking machinery controls the availability of neurotransmitter receptors at the plasma membrane, which is a critical determinant of synaptic strength. Metabotropic γ amino-butyric acid (GABA) type B receptors (GABA(B)Rs) are neurotransmitter receptors that modulate synaptic transmission by mediating the slow and prolonged responses to GABA. GABA(B)Rs are obligatory heteromers constituted by two subunits, GABA(B)R1 and GABA(B)R2. GABA(B)R1a and GABA(B)R1b are the most abundant subunit variants. GABA(B)R1b is located in the somatodendritic domain whereas GABA(B)R1a is additionally targeted to the axon. Sushi domains located at the N-terminus of GABA(B)R1a constitute the only difference between both variants and are necessary and sufficient for axonal targeting. The precise targeting machinery and the organelles involved in sorting and transport have not been described. Here we demonstrate that GABA(B)Rs require the Golgi apparatus for plasma membrane delivery but that axonal sorting and targeting of GABA(B)R1a operate in a pre-Golgi compartment. In the axon GABA(B)R1a subunits are enriched in the endoplasmic reticulum (ER), and their dynamic behavior and colocalization with other secretory organelles like the ER-to-Golgi intermediate compartment (ERGIC) suggest that they employ a local secretory route. The transport of axonal GABA(B)R1a is microtubule-dependent and kinesin-1, a molecular motor of the kinesin family, determines axonal localization. Considering that progression of GABA(B)Rs through the secretory pathway is regulated by an ER retention motif our data contribute to understand the role of the axonal ER in non-canonical sorting and targeting of neurotransmitter receptors.
Conflict of interest statement
Figures


References
-
- Horton AC, Ehlers MD (2004) Secretory trafficking in neuronal dendrites. Nat Cell Biol 6: 585–591. - PubMed
-
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84: 835–867. - PubMed
-
- Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, et al. (1998) Intracellular retention of recombinant GABAB receptors. J Biol Chem 273: 26361–26367. - PubMed
-
- Margeta-Mitrovic M, Jan YN, Jan LY (2000) A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27: 97–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

